The group of Ralph Schermuly is committed to translational research in heart and lung diseases focussing on pulmonary vascular disease and right heart hypertrophy. Both clinical conditions are characterized by increased proliferation and resistance to apoptosis of vascular smooth muscle cells and endothelial cells in the case of pulmonary hypertension, and inflammation, matrix synthesis and compensated/decompensated structure and function in the case of right ventricular hypertrophy. Examples of his “from bench to bedside” research include preclinical investigations of inhaled prostanoids, phosphodiesterase inhibitors, soluble guanylate cyclase activators providing the basis for worldwide approval of inhaled iloprost, inhaled treprostinil, oral sildenafil, oral tadalafil and riociguat for treatment of pulmonary arterial hypertension. Additional examples of the translational research include inhibition of receptor tyrosine kinases (for both Imatinib and Seralutinib, Phase 2 Trials have been finished and Phase 3 trials have been initiated).
Against this background, the Schermuly group focusses on:
1) Cell Culture and Organ Models for Pulmonary Hypertension
Cell culture models are fundamental in studying the cellular and molecular mechanisms underlying PH. These models primarily involve the use of pulmonary artery smooth muscle cells (PASMCs), endothelial cells (PAECs), and fibroblasts. These cells can be isolated from human or animal pulmonary arteries and cultured in vitro to investigate specific pathways and responses to various stimuli.
2) Organ Models
Organ models, including organoids and tissue-engineered constructs, represent a significant advancement in PH research. These models aim to replicate the three-dimensional architecture and microenvironment of the pulmonary artery, providing a more accurate representation of in vivo conditions.
a.) Precision Cut Lung/Heart Slices: In pulmonary vascular disease, hemodynamics, extracellular matrix, paracrine factors, and intra-cellular crosstalk are essential for vascular cell maturation and maintaining homeostasis. In-vitro culture platforms such as organoid and microfluidics-based systems only recapitulate a few physiological properties while failing to mimic the other aspects of lung physiology. Thus, whole organ decellularization becomes more critical in understanding processes often attributed to the vasculature's pathological responses. The Schermuly group provides native and decellularized Precision-cut lung/heart slices as well as whole organs of mice/rat and humans to evaluate drug/multi-drug therapy approaches and provide additional insight into hemodynamic forces, extracellular matrix remodeling, and pulmonary matrix-cellular interaction in PH under physiologically relevant conditions.
b.) Ex Vivo Lung Perfusion (EVLP) Models: EVLP systems maintain isolated lungs in a near-physiological state, allowing for detailed study of pulmonary hemodynamics and gas exchange. These models are particularly useful for testing the effects of novel therapeutic agents on pulmonary vascular resistance and right ventricular function in a controlled environment. EVLP combined with lung culture provides a powerful platform for ex-vivo disease modeling in pulmonary hypertension.

Isolated ventilated and perfused mouse lung
3) Animal Models of Pulmonary Hypertension
Animal models play a crucial role in advancing our understanding of pulmonary hypertension (PH) and in the development of effective therapies. These models allow researchers to study the pathophysiological mechanisms underlying PH in a whole-organism context, which includes the interactions between different cell types, tissues, and organ systems that are difficult to replicate in vitro. Several animal models, each with unique advantages and limitations, have been developed to mimic the various etiologies and stages of PH.
a.) Monocrotaline (MCT)-Induced Model: This model involves a single injection of monocrotaline, a toxic alkaloid, which induces severe pulmonary vascular injury leading to PH. The MCT model is widely used due to its simplicity and reproducibility. It mimics aspects of human PH, such as pulmonary arterial remodeling, right ventricular hypertrophy, and increased pulmonary arterial pressure.
b.) Chronic Hypoxia Model: Exposure to chronic hypoxia (10% oxygen) induces PH in rodents by promoting pulmonary vascular remodeling and vasoconstriction. This model is particularly relevant for studying hypoxia-induced pulmonary hypertension, such as that seen in high-altitude residents or patients with chronic obstructive pulmonary disease (COPD).
c.) Sugen 5416/Hypoxia (SuHx) Model: Combining a single injection of the vascular endothelial growth factor receptor inhibitor Sugen 5416 with chronic hypoxia, the SuHx model results in severe and progressive PH with features resembling human idiopathic pulmonary arterial hypertension (IPAH). This model is valuable for studying the interplay between genetic predisposition and environmental factors in PH.
d.) Genetically Modified Models: Transgenic and knockout mice, with specific genes altered or deleted, provide insights into the genetic factors contributing to PH. For instance, mice lacking the BMPR2 gene, which is commonly mutated in familial forms of PH, develop spontaneous PH and are used to study the genetic underpinnings of the disease.
e.) PAB: Pulmonary arterial banding (PAB) allows the mechanical induction of right heart hypertrophy due to the increased vascular resistance. In contrast to monocrotaline- or hypoxia-induced RVH, this RVH allows a targeted analysis of the development mechanism of right heart hypertrophy as a primary disease and not as a secondary phenomenon, as in the above-mentioned models.
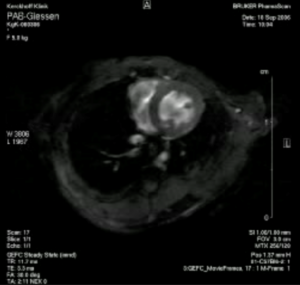
Echocardiography of right heart hypertrophy in a mouse model of pulmonary arterial banding
f.) PVB: PH group 2 accounts for the largest proportion of patients with clinically relevant PH worldwide. In comparison to models mimicking PH group 1, hardly any models for PH group 2 have been able to establish themselves in the scientific community to date. Therefore, we have established a model of group 2 secondary pulmonary hypertension due to partial pulmonary vein occlusion (PVPV) in one of our latest studies.
g.) high fat/L-NAME: To date, there is no approved therapy for HFpEF-associated PH. Against this background, it is of great importance to find suitable treatment options for HFpEF-associated PH. To overcome this problem, recently, a mouse model was published and validated in which HFpEF and relevant pulmonary congestion with signs of PH were detectable. In this mouse model, the animals are fed a high-fat diet and the NO-synthase inhibitor L-NAME is added to the drinking water. We are currently utilizing this 2-hit model with a mechanical and a metabolic stressor in one of our projects, which may pave the way for new treatment options for HFpEF-PH.
4.) Omic Technologies in Pulmonary Hypertension Research
The advent of omic technologies has revolutionized biomedical research, providing comprehensive insights into the molecular underpinnings of diseases, including pulmonary hypertension (PH). By enabling the simultaneous analysis of large-scale data across different biological layers, such as genomics, transcriptomics, proteomics, and metabolomics, these technologies have significantly advanced our understanding of PH pathogenesis, progression, and potential therapeutic targets.
Genomics
a.) Genome-Wide Association Studies (GWAS): GWAS have identified numerous genetic loci associated with PH, particularly in idiopathic and heritable forms of the disease. For example, mutations in the BMPR2 gene have been strongly linked to familial PH, highlighting the importance of genetic predisposition in disease development.
b.) Whole-Exome and Whole-Genome Sequencing: These approaches provide a comprehensive analysis of the coding and non-coding regions of the genome, respectively. By identifying rare and novel genetic mutations, these techniques have uncovered new genes and pathways implicated in PH, offering potential targets for therapeutic intervention.
Transcriptomics
a.) Differential Gene Expression Analysis: By comparing the transcriptomes of PH patients and healthy controls, researchers have identified differentially expressed genes involved in vascular remodeling, inflammation, and cellular proliferation, which are key processes in PH pathogenesis.
b.) Single-Cell RNA Sequencing (scRNA-seq): This technique provides high-resolution insights into the heterogeneity of cell populations within the pulmonary vasculature. scRNA-seq has revealed distinct cellular subpopulations and their specific gene expression profiles, contributing to a better understanding of the cellular landscape and intercellular communication in PH.
Proteomics
a.) Mass Spectrometry (MS)-Based Proteomics: MS has been instrumental in identifying protein biomarkers and altered signaling pathways in PH. Proteomic profiling of plasma, lung tissue, and pulmonary artery cells from PH patients has uncovered dysregulated proteins that play critical roles in disease progression.
b.) Post-Translational Modifications (PTMs): Understanding PTMs, such as phosphorylation, acetylation, methylation and ubiquitination, is crucial for elucidating the functional regulation of proteins in PH. Proteomic studies have identified altered PTM patterns in key signaling proteins, offering insights into the mechanisms driving vascular remodeling and right ventricular dysfunction.
Metabolomics
a.) Metabolic Profiling: Metabolomic studies have identified altered metabolic pathways in PH, such as dysregulated glycolysis, impaired mitochondrial function, and altered lipid metabolism. These metabolic alterations are critical for understanding the bioenergetic and biosynthetic demands of proliferating pulmonary artery cells.
b.) Biomarker Discovery: Metabolomic profiling of blood and urine samples from PH patients has led to the identification of novel metabolic biomarkers that could be used for early diagnosis, disease monitoring, and therapeutic response assessment.
Kinomics
Peptide-based kinase activity profiling via the PamStation fully completes the set of our portfolio of expertise as mentioned above. This methodology represents an important step in the functional analysis of protein kinases and their involvement in the pathology of PH. In addition, it allows the identification of new potential drug targets and opens the field of drug repurposing of kinase inhibitors.

Kinase activity within a given samples (e.g. cell lysate) can be determined by using the PamStation system with PamChips which are coated with phosphorylable substrate peptides
Prof. Dr. rer. nat. Ralph Schermuly
Chair for Pulmonary Pharmacotherapy
Department of Internal Medicine
Justus-Liebig-University Giessen
University of Giessen and Marburg Lung Center (UGMLC)
Excellence Cluster Cardio Institute (CPI) Member of the German Lung Center (DZL)
Institute of Lung Health (ILH)
ILH platform leader Drug Repurposing Hub
Aulweg 130
35392 Giessen
Tel: +49 641 99 42428 (Assistant: Ms Daniela Weber)
Tel: +49 641 99 42475 (Assistant: Ms Patricia Michael)
Tel: +49 641 99 42422 (Assistant: Ms Elizabeta Krstic)
Tel: +49 (0) 641 99 42420
Fax:+49 (0) 641 99 42419
Email: Ralph.Schermuly@innere.med.uni-giessen.de
https://cpi-online.de
https://ilh-giessen.de/en
ORCID ID 0000-0002-5167-6970
Post Docs
Dr. Argen Mamazhakypov, Dr. Nabham Rai, Dr. Mazen Shihan, Dr. Astrid Weiß, Dr. Swathi Veeroju
Doctoral Students
Tobiah Antoine, Leonhard Blumrich, Jaron Lukas Bornhäuser, Vivian Feichtenschlager, Tom Feifarek, Muriel Fischer, Giuseppina Di Nolfo, Vandna Sapehia, Tim Schatull, Anna Schumacher, Kunwarveer Singh
Technical Assistants
Julia Baldauf, Andreas Hecker, Carina Lepper, Christina Vroom