Prof. Dr. Grazyna Kwapiszewska
Grazyna Kwapiszewska is a professor at the Institute for Lung Health (ILH) of Justus Liebig University (JLU) in Giessen, Germany, where she leads the research group “Aberrant Remodeling and Regeneration in Chronic Lung Diseases.” She is also the director of Ludwig Boltzmann Institute for Lung Vascular Research (LBI-LVR) in Graz, Austria and Associated Professor for “Vascular Remodeling” at the Department of Physiology, Medical University of Graz, Austria. She is also a speaker of the Austrian Science Fund (FWF) funded graduate school Immune Modulation in Respiratory Diseases (RespImmun).
Grazyna Kwapiszewska was born in Poland. After receiving her Masters (MSc) in Biotechnology from Adam Mickiewicz University, Poznan, Poland she moved to Germany and finished her PhD and postdoctoral training at the Graduate Program Molecular Biology and Medicine of the Lung (MBML) and Pathology/Internal Medicine Department of the JLU. From 2005 she served as teaching associate and later as deputy director of Graduate Program MBML. In 2008 she was appointed as a faculty member at the University of Giessen and Marburg Lung Center (UGMLC), Giessen, Germany. In 2011, she moved to Graz, Austria, to run an independent research group and served as deputy director of LBI-LVR. In 2015 she has habilitated in Molecular Pathology at the Medical University of Graz. From 2017 she became the director of LBI-LVR, and in 2020 received an endowed professorship at the Department of Physiology, Medical University of Graz. Dr. Kwapiszewska is on the editorial board of several journals including: AJRCMB, AJP-Lung, and AJP-Cell Physiology and is serving as a reviewer of numerous funding agencies and scientific articles. She has received several national and international awards for her scientific accomplishments. She has published 105 research articles (Date: Sept. 2021) in well-renowned scientific journals, including: Circulation, Circulation Research, American Journal of Respiratory and Critical Care Medicine, European Respiratory Journal, Nature Communications, American Journal of Molecular and Cellular Biology, and American Journal of Physiology-Lung.
Scientific Program of the Professorship
Pulmonary vascular diseases have multifactorial pathobiology, characterized by aberrant phenotypes of endothelium, smooth muscle cells and adventitial fibroblasts. These pathologic changes cause remodeling of the pulmonary arteries (PAs) not only in the idiopathic form of pulmonary arterial hypertension (PAH), but also in chronic lung diseases (CLD). The Kwapiszewska team has deciphered several molecular and cellular mechanisms underlying the pathobiology of pulmonary vascular remodeling in different forms of CLD associated to pulmonary hypertension (PH). We discovered dysregulation in the extracellular matrix and basement membrane composition of the PAs of CLD-PH patients. Furthermore, we identified transcription factors, which - in a compartment-specific manner - lead to the abnormal proliferation of lung vascular cells and their interaction with immunomodulatory processes.
The Kwapiszewska team focuses on the “vascular point of view on chronic lung diseases”. Chronic Lung Diseases (CLDs), such as COPD and pulmonary fibrosis, are an increasing cause of death in the aging population. As vascular involvement in these diseases worsens patient outcome, our team aims to unravel the compartment-specific role of mesenchymal cells/disturbance of basement components and their interaction with immune processes in the pathobiology of CLD and their vascular abnormalities. Based on our previous experience with lineage tracing technology, single-cell analysis and multi-color fluorescent microscopy to dissect the heterogeneity of the lung vascular abnormalities, we aim to extend these methodological approaches to characterize the cellular and molecular mechanisms leading to the formation and progression of vascular lesions in the context of CLD, as well as non-resolving parenchymal abnormalities post pneumonia/ARDS. The role of basement membrane and basement membrane-derived matrikines in these pathogenetic sequelae will be particularly addressed. In a translational aspect, we aim to identify new vascular biomarkers predicting patients’ outcome and to identify and verify novel treatment approaches derived from the “vascular point of view” of these diseases, as depicted in the subsequent figure:
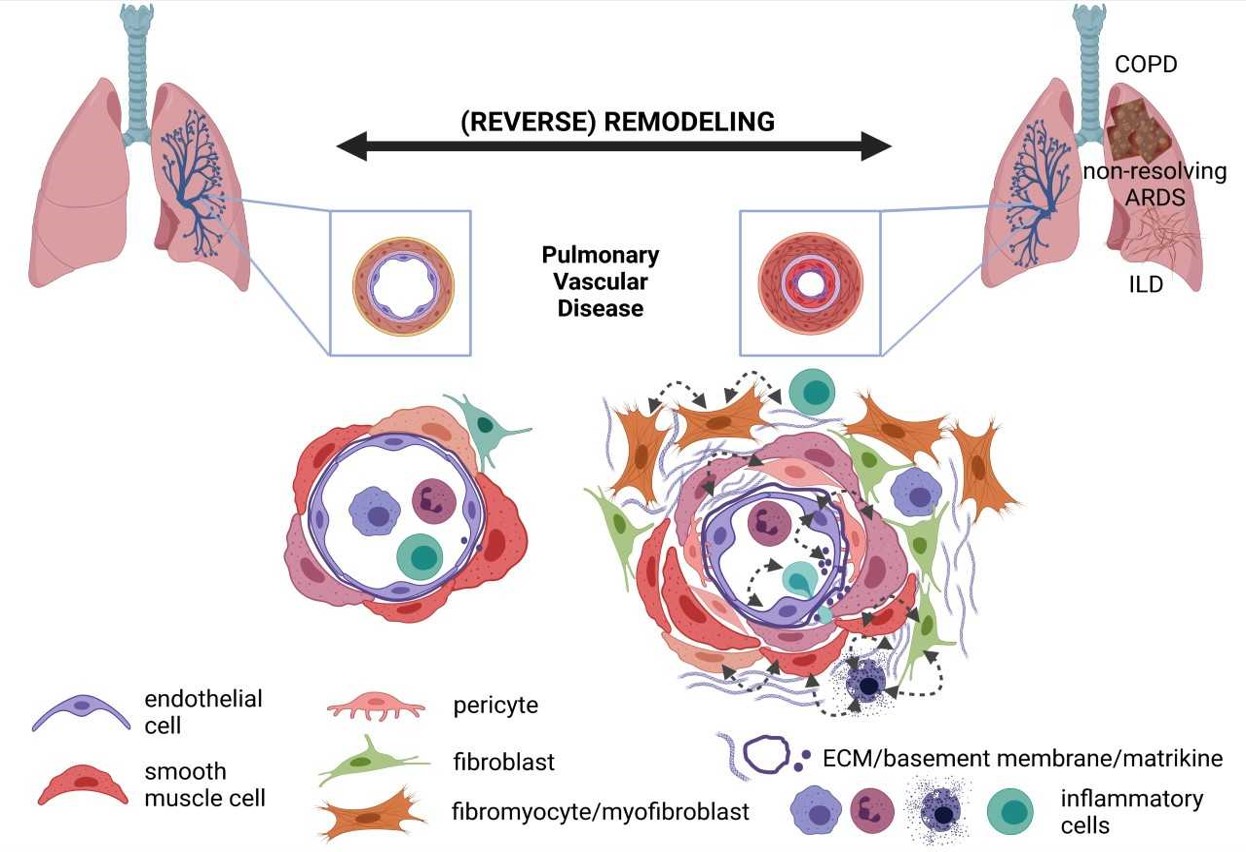

The following goals will be addressed:
Basement membrane interaction with structural and immune cells: intercellular and cell-matrix cross-talk:
- Determination which inflammatory cell subpopulations are the source of enzymes liberating matrikines and how matrikines modulate the inflammatory landscape
- Mapping of the spatiotemporal architecture of the immune cells and their relation with basement membrane components in the vessel wall
- Delineating the functional effects of basement membrane components and matrikines on EC and PASMC behavior (in vitro studies) and pulmonary vascular remodeling (experimental studies)
- Employing pro- and anti-matrikine strategies to modulate the lung vascular abnormalities in experimental CLD and post-injury models
- Phenotypic stratification of CLD patients according to their matrikine fingerprints – association with morbidity and mortality as accessible via the DZL Data Warehouse
Heterogeneity of the cellular components in the pulmonary vascular wall and their role in CLD initiation/progression and associated vascular abnormalities:
- Building a high-resolution, genome-wide atlas of epigenetic modifications of the various (peri-)vascular cell types and subpopulations, encompassing histone modifications, chromatic accessibility and DNA methylation; association of these profiles with the remodelling process in CLD and non-resolving post-injury lung abnormalities
- Combining the data sets of transcriptional and epigenetic profiling to reconstruct the trajectory of the various (sub)-populations and determine their fate upon CLD- and post-injury vascular remodeling
- Lineage tracing of novel vascular resident cell subpopulations, identified in the previous studies
- Modulation of immune cell composition of the (peri)-vascular compartment to re-establish of the vascular homeostasis in CLD-associated vascular abnormalities
Contact
Email: Grazyna.Kwapiszewska@lvr.lbg.ac.at
Ten most important publications
- Birnhuber A, Fliesser E, Gorkiewicz G, Zacharias M, Seeliger B, David S, Welte T, Schmidt J, Olschewski H, Wygrecka M, Kwapiszewska G. Between inflammation and thrombosis - endothelial cells in COVID-19. Eur Respir J. 2021 May 13:2100377. (IF: 16.6)
- Biasin V, Crnkovic S, Sahu-Osen A, Birnhuber A, El Agha E, Sinn K, Klepetko W, Olschewski A, Bellusci S, Marsh LM, Kwapiszewska G. PDGFRα and αSMA mark two distinct mesenchymal cell populations involved in parenchymal and vascular remodeling in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2020 Apr 1;318(4):L684-L697. doi: 10.1152/ajplung.00128.2019. (IF: 5.4)
- Jandl K, Marsh LM, Hoffmann J, Mutgan AC, Baum O, Bloch W, Thekkekara-Puthenparampil H, Kolb D, Sinn K, Klepetko W, Heinemann A, Olschewski A, Olschewski H, Kwapiszewska G. Basement Membrane Remodeling Controls Endothelial Function in Idiopathic Pulmonary Arterial Hypertension. Am J Respir Cell Mol Biol. 2020 Jul;63(1):104-117. doi: 10.1165/rcmb.2019-0303OC. (IF: 6.9)
- Birnhuber A, Crnkovic S, Biasin V, Marsh LM, Odler B, Sahu-Osen A, Stacher-Priehse E, Brcic L, Schneider F, Cikes N, Ghanim B, Klepetko W, Graninger W, Allanore Y, Eferl R, Olschewski A, Olschewski H, Kwapiszewska G. IL-1 receptor blockade skews inflammation towards Th2 in a mouse model of systemic sclerosis. Eur Respir J. 2019 Sep 29;54(3). (IF: 16.6)
- Biasin V, Wygrecka M, Bärnthaler T, Jandl K, Jain PP, Bálint Z, Kovacs G, Leitinger G, Kolb-Lenz D, Kornmueller K, Peters F, Sinn K, Klepetko W, Heinemann A, Olschewski A, Becker-Pauly C, Kwapiszewska G. Docking of Meprin α to Heparan Sulphate Protects the Endothelium from Inflammatory Cell Extravasation. Thromb Haemost. 2018 Sep 20. doi: 10.1055/s-0038-1670657. (IF:5)
- Marsh LM, Jandl K, Grünig G, Foris V, Bashir M, Ghanim B, Klepetko W, Olschewski H, Olschewski A, Kwapiszewska G. The inflammatory cell landscape in the lungs of patients with idiopathic pulmonary arterial hypertension. Eur Respir J. 2018 Jan 25;51(1). (IF: 16.6)
- Crnkovic S, Marsh LM, El Agha E, Voswinckel R, Ghanim B, Klepetko W, Stacher-Priehse E, Olschewski H, Bloch W, Bellusci S, Olschewski A, Kwapiszewska G. Resident cell lineages are preserved in pulmonary vascular remodeling. J Pathol. 2018 Apr;244(4):485-498. (IF:6)
- Kwapiszewska G, Gungl A, Wilhelm J, Marsh LM, Puthenparampil HT, Sinn K, Didiasova M, Klepetko W, Kosanovic D, Schermuly RT, Wujak L, Weiss B, Schaefer L, Schneider M, Kreuter M, Olschewski A, Seeger W, Olschewski H, Wygrecka M. Transcriptome profiling reveals the complexity of pirfenidone effects in IPF. Eur Respir J. 2018 Aug 30. pii: 1800564. (IF: 16.6)
- Hoffmann J, Marsh LM, Pieper M, Stacher E, Ghanim B, Kovacs G, König P, Wilkens H, Haitchi HM, Hoefler G, Klepetko W, Olschewski H, Olschewski A, Kwapiszewska G. Compartment-specific expression of collagens and their processing enzymes in intrapulmonary arteries of IPAH patients. Am J Physiol Lung Cell Mol Physiol. 2015 May 15;308(10):L1002-13. (IF: 5.4)
- Hoffmann J, Wilhelm J, Marsh LM, Ghanim B, Klepetko W, Kovacs G, Olschewski H, Olschewski A, Kwapiszewska G. Distinct differences in gene expression patterns in pulmonary arteries of COPD and IPF patients. Am J Respir Crit Care Med. 2014 Jul 1;190(1):98-111. (IF: 21)

