Menu
close
Elie El Agha is appointed as Professor at the Institute for Lung Health (ILH), Justus-Liebig University Giessen (JLU), Germany, where he leads the research group “Pathogen-Induced Lung Injury and Repair”. He is the director of the international graduate program Molecular Biology and Medicine of the Lung (MBML) and also faculty member and co-leader of Area 3 on “Morphogenesis, Remodeling & Regeneration” at the excellence cluster Cardio-Pulmonary Institute (CPI). He is also a principal investigator and coordinator of Area C on “The Mesenchymal Cell: An Active and Powerful Modulator of the Pulmonary Scaffold into the Spatial Context” at the Diffuse Parenchymal Lung Disease (DPLD) disease area of the German Center for Lung Research (DZL).
Elie El Agha was born in Saida, Lebanon. After receiving his Bachelor degree (BSc) in Biochemistry from the Lebanese University, Beirut, Lebanon, he moved to Sweden where he did his Master degree (MSc) in molecular biology at Skövde University. For his Master thesis, he received a fellowship to work in the lab of Prof. Hans Jörnvall, Department of Medical Biochemistry and Biophysics at the Karolinska Institute, Stockholm, Sweden. In 2009, he moved to JLU, Germany, where he did his PhD and postdoctoral training on lung development and repair in the lab of Prof. Saverio Bellusci, Chair for Lung Matrix Remodeling at the Excellence Cluster Cardio-Pulmonary System (ECCPS). For his early career achievements, he was awarded two start-up grants and one intramural fund in addition to other prizes and awards. He is also a member of the editorial board of the American Journal of Physiology – Lung Cellular and Molecular Physiology, and served as guest editor at Canadian Respiratory Journal and Frontiers in Cell and Developmental Biology. He published 37 scientific articles (Date 15th Jan 2021) in reputable scientific journals in the fields of stem cells, development, cell biology and pulmonary research such as Cell Stem Cell, Nature Communications, Developmental Cell, EMBO Journal, Cell Reports, Stem Cells, Journal of Pathology, Development, PLoS Pathogens, BMC Biology, American Journal of Physiology – Lung Cellular and Molecular Physiology, Developmental Dynamics and others.
The El Agha lab focuses on the cellular and molecular mechanisms mediating lung development and repair, particularly the interaction between mesenchymal niche cells and their epithelial partners along the proximo-distal axis in the respiratory tree. The lab is interested in the involvement of developmental signaling mechanisms in disease progression versus resolution, especially in the context of pathogen-induced lung injury.
Lung injury resulting from bacterial or viral infections represents a serious threat to global health. For instance, influenza A virus and coronavirus outbreaks can lead to pandemics resulting in millions of deaths worldwide. Pneumonia is one of the most common causes of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). The latter is defined by the acute onset of noncardiogenic pulmonary edema, hypoxemia and the need for mechanical ventilation. Among the prominent features of ARDS are diffuse alveolar damage and collagen deposition. Pathogen-induced lung diseases are associated with a high socio-economic burden and they mostly lack preventive or curative therapy. In this context, available therapeutic options for affected patients are predominantly supportive and are incapable of efficiently inducing regeneration in damaged lungs. Importantly, pathogen-induced pneumonia per se not only leads to acute life-threatening conditions such as ARDS but also 1) acts as a secondary hit that contributes to the initiation of fatal and progressive lung diseases such as idiopathic pulmonary fibrosis (IPF) and 2) represents a comorbidity that contributes to exacerbations such as in chronic obstructive pulmonary disease (COPD) and IPF. Thus, acute exacerbations and hospitalization due to pathogen-induced lung injury represent an increased risk in patients with chronic lung disease. Therefore, better understanding of the cellular and molecular cues that mediate pathogen-induced lung injury and the associated repair mechanisms is an urgent clinical need. In this regard, there is a general consensus that developmental processes of lung morphogenesis are recapitulated or at least mimicked in lung repair during adult life.
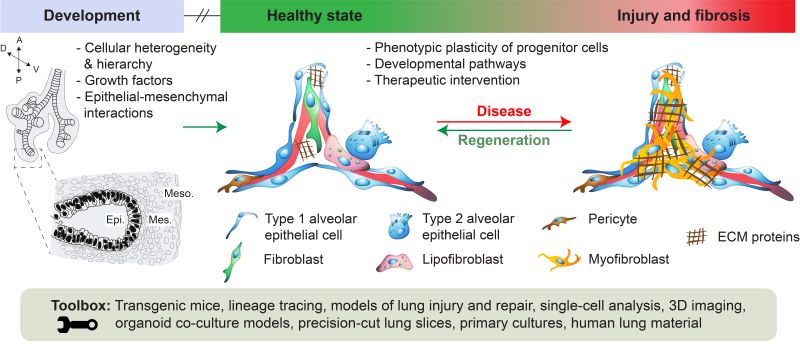
Figure 1: Overview of the research topics addressed in the El Agha lab
In recent years, we investigated basic mechanisms of lung organogenesis, including the cellular and molecular signaling cues that govern the behavior of progenitor cells during embryonic and postnatal lung development. We focused on the involvement of growth factor signaling in mediating the crosstalk between epithelial and mesenchymal cells and contributed to delineating the lineage tree of the lung mesenchyme not only during embryonic and postnatal lung development but also during lung disease in the adult stage. We demonstrated that phenotypic plasticity of mesenchymal cells is at the heart of both disease progression and resolution, and that reconstituting the mesenchymal niche is critical for proper lung regeneration. Among the pathways of interest are FGF, hedgehog, WNT, PPARg, TGFb and PDGFRa signaling pathways. Against this background, the professorship for Pathogen-Induced Lung Injury and Repair aims to establish a “Repair Blueprint” for the lung by focusing on the following aspects:
Prof. Elie El Agha, Ph.D.
Professor for Pathogen-Induced Lung Injury and Repair at the Institute for Lung Health (ILH)
Director of the international graduate program Molecular Biology and Medicine of the Lung (MBML)
Cardio-Pulmonary Institute (CPI)
Member of the German Center for Lung Research (DZL)
Universities of Giessen and Marburg Lung Center (UGMLC)
Justus-Liebig University Giessen
Aulweg 132
35392 Giessen
Tel: +49 (0) 641 99 36481 (office)
Tel: +49 (0) 641 99 36482 (lab)
Fax: +49 (0) 641 99 36429
Email:
elie.el-agha@innere.med.uni-giessen.de
Web:
https://www.uni-giessen.de/fbz/fb11/forschung/graduierte/mbml

Georgios Kiliaris, Ali Khadim, Mahsa Zabihi, Mahtab Shahriari Felordi
Hannah Hofmann
Dr. Sezin Czarnecki
Alena Moiseenko, Vahid Kheirollahi, Felix Schwind, Xuran Chu, Laith Salama