Dr. Ana Ivonne Vazquez-Armendariz, Ph.D
Ana Ivonne Vazquez-Armendariz is appointed as Junior Group Leader at the Institute for Lung Health (ILH), Justus-Liebig University Giessen (JLU), Germany, where she leads the research group “Lung organoids and disease modeling”. She is a committee member of the international graduate program Molecular Biology and Medicine of the Lung (MBML), member of the excellence cluster Cardio-Pulmonary Institute (CPI) and the German Center for Lung Research (DZL).
Ana Ivonne Vazquez-Armendariz was born in Monterrey, Mexico. She obtained her bachelor degree on Clinical Biochemistry with honors from the Autonomous University of Nuevo Leon in Mexico in 2009. After graduation, she was awarded with a scholarship by the Mexican National Council of Science and Technology (CONACYT) to pursue her postgraduate degree in Germany. Vazquez-Armendariz completed her master degree in Molecular Medicine at the University of Medicine Charité, Germany in 2012. Vazquez-Armendariz early research focused on Mycobacterial infections, including the study of the immune response of diabetic patients with tuberculosis and crosstalk between alveolar epithelial cells and alveolar macrophages during Mycobacterial Infection. In 2013, she moved to Giessen where she did her PhD and postdoctoral training at the JLU Giessen in the lab of Prof. Susanne Herold. During this period, her research work centered on the establishment and refinement of three-dimensional (3D) murine bronchioalveolar lung organoid cultures obtained from adult somatic stem cells for modeling of lung development and disease.
The Vazquez-Armendariz lab primarily focuses on the use of three-dimensional lung organoid systems from adult somatic stem cells and induced pluripotent stem cells (iPSCs) for modeling lung development and disease and establishing novel therapeutic strategies. In particular, the molecular crosstalk between the cellular players of the epithelial-mesenchymal-myeloid unit during lung development, infection, injury and repair will be in the focus. The lab will elucidate cell-specific molecular signaling pathways involved in disease resolution, especially in the context of pathogen-induced lung injury.
In the context of injury, infection of BALO epithelium with different strains of IAV has been shown to induce viral replication and spread, and an antiviral immune response. Additionally, microinjection of IAV into BALO airway-like structures recapitulates proximal-to-distal spread of the infection and causes substantial loss of AEC in the infected alveolar-like areas, thus, modeling this particular aspect of the in vivo situation of IAV pneumonia. Moreover, BALO can be supplemented with resident lung immune cells (e.g., alveolar macrophages) or tumor cells that engraft into the BALO, and with endothelial cells that form vascular tubes and a 3D capillary network and directly interact with AEC I at the alveolar interface, representing another level of complexity to be studied in the context of lung development, viral infection/injuries and lung cancer.
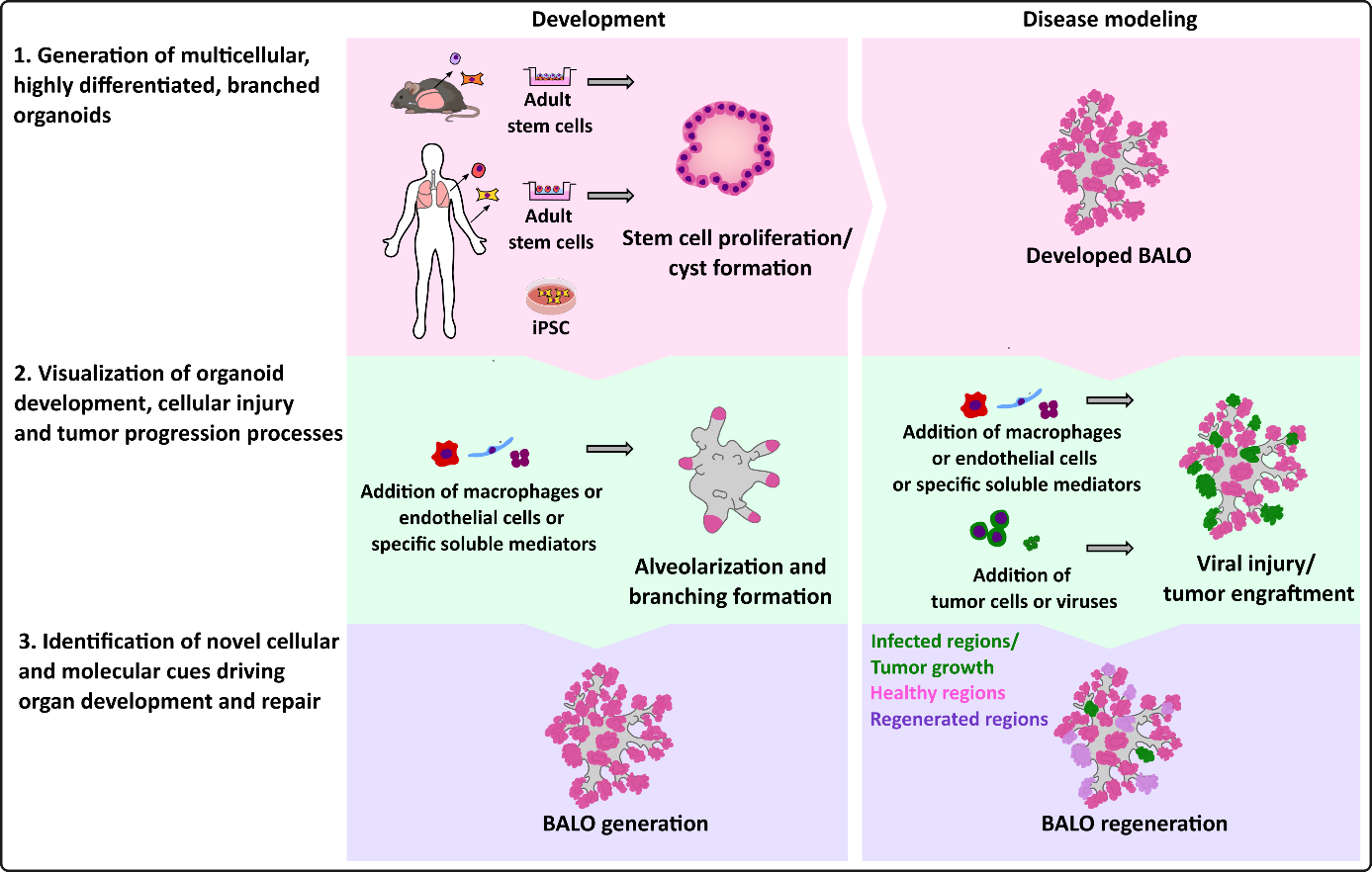
Figure 1: Overview of research topics addressed in Vazquez-Armendariz group
Therefore, the junior research group for “Lung organoids and disease modeling” aims to employ different variants of the lung organoid technique to model diseases that affect the bronchoalveolar compartment of the lung by focusing on the following aims:
- To develop multicellular, highly differentiated, branched human lung organoid systems that closely resemble the lung architecture, with respect to its epithelial, mesenchymal, endothelial and immunological feature. Here, the mouse cell focus will be complemented by a human cell focus.
- To visualize organoid regeneration after organoid injury induced by viral infection (in particular IAV and SARS-CoV-2, in cooperation with other ILH Faculty members) or other insults, as well as lung cancer, at high resolution and over time by microscopic analysis.
- To determine the role of gene-specific functions in BASC, endothelial cells, mesenchymal cells and/or leukocytes constituting the alveolar niche during development, infection, injury and repair by i) employing cells with gain- or loss-of-function mutations (possibly combined with gene reporters) originating from transgenic mice, and ii) employing gene silencing and CRISPR-Cas9 gene editing techniques to specifically manipulate the cells before entering them into the organoid (mouse and human cells).
- To monitor cellular interactions happening during injury and repair upon microinjection of relevant cell types into lung organoids such as tumor cells, myeloid and lymphocyte cell populations, and particularly, differently polarized macrophages. In particular, the impact of these cells in disease resolution mechanisms will be studied.
- To confirm in vivo the validity of novel findings made with the organoid models by cooperating with ILH Faculty members performing experimental studies in the various mouse lung disease models and in human viable precision cut lung slices (PCLS), as well as organotypic, highly differentiated primary human lung cell cultures (airway, alveolar).
Contact
Dr. Ana Ivonne Vazquez-Armendariz, Ph.D
Group leader for lung organoids and disease modeling
Tutor of the international graduate program Molecular Biology and Medicine of the Lung (MBML)
Institute for lung health (ILH)
Cardio-Pulmonary Institute (CPI)
Member of the German Center for Lung Research (DZL)
Universities of Giessen and Marburg Lung Center (UGMLC)
Justus-Liebig University Giessen
Aulweg 132
35392 Giessen
Tel: +49 (0) 641 99 36402 (office)
Tel: +49 (0) 641 99 36406 (lab)
Fax: +49 (0) 641 99 36406
Email:
Ana.I.Vazquez-Armendariz@innere.med.uni-giessen.de
Ten most important publications
- Vazquez-Armendariz AI, Seeger W, Herold S, El Agha E. Protocol for the generation of murine bronchiolospheres. STAR Protoc. 2021 2(2):100594.
- Vazquez-Armendariz AI, Herold S. From clones to buds and branches: The use of lung organoids to model branching morphogenesis ex vivo. Front Cell Dev Biol. 2021 9:631579 (IF 5.2).
- Ahmadvand N, Khosravi F, Lingampally A, Wasnick R, Vazquez-Armendariz AI, Carraro G, Heiner M, Rivetti S, Lv Y, Wilhelm J, Gunther A, Herold S, Al Alam D, Chen C, Minoo P, Zhang JS, Bellusci S. “Identification of a novel subset of alveolar type 2 cells enriched in PD-L1 and expanded following pneumonectomy”. Eur Respir J. 2021 Apr 16:2004168. doi: 10.1183/13993003.04168-2020 (IF 12.3).
- Hoek A, Maibach K, Özmen E, Vazquez-Armendariz AI, Mengel JP, Hain T, Herold S, Goesmann A. WASP: a versatile, web-accessible single cell RNA-Seq processing platform. BMC Genomics. 2021 Mar 18;22(1):195. doi: 10.1186/s12864-021-07469-6 (IF 4).
- Vazquez-Armendariz AI, Heiner M, El Agha E, Salwig I, Hoek A, Hessler MC, Shalashova I, Shrestha A, Carraro G, Mengel JP, Günther A, Morty RE, Vadász I, Schwemmle M, Kummer W, Hain T, Goesmann A, Bellusci S, Seeger W, Braun T, Herold S. Multilineage murine stem cells generate complex organoids to model distal lung development and disease. EMBO J. 2020 39(21):e103476 (IF 11.6).
- Moiseenko A, Vazquez-Armendariz AI, Kheirollahi V, Chu X, Tata A, Rivetti S, Günther S, Lebrigand K, Herold S, Braun T, Mari B, De Langhe S, Kwapiszewska G, Günther A, Chen C, Seeger W, Tata PR, Zhang JS, Bellusci S, El Agha E. Identification of a Repair-Supportive Mesenchymal Cell Population during Airway Epithelial Regeneration. Cell Rep. 2020 33(12):108549 (IF 9.4).
- Kheirollahi V, Wasnick RM, Biasin V, Vazquez-Armendariz AI, Chu X, Moiseenko A, Weiss A, Wilhelm J, Zhang JS, Kwapiszewska G, Herold S, Schermuly RT, Mari B, Li X, Seeger W, Günther A, Bellusci S, El Agha E. “Metformin Induces Lipogenic Differentiation in Myofibroblasts to Reverse Lung Fibrosis”. Nat Commun. 2019 Jul 5;10(1):2987. doi: 10.1038/s41467-019-10839-0 (IF 14.9).
- Salwig I, Spitznagel B, Vazquez-Armendariz AI, Khalooghi K, Guenther S, Herold S, Szibor M, Braun T. Bronchioalveolar stem cells are a main source for regeneration of distal lung epithelia in vivo. EMBO J. 2019 38(12):e102099 (IF 11.6).
- Schmoldt C, Vazquez-Armendariz AI, Shalashova I, Selvakumar B, Bremer CM, Peteranderl C, Wasnick R, Witte B, Gattenlöhner S, Fink L, Vadász I, Morty RE, Pleschka S, Seeger W, Günther A, Herold S. “IRE1 Signaling As a Putative Therapeutic Target in Influenza Virus-induced Pneumonia”. Am J Respir Cell Mol Biol. 2019 Oct;61(4):537-540. doi: 10.1165/rcmb.2019-0123LE (IF 6.9).
- Quantius J, Schmoldt C, Vazquez-Armendariz AI, Becker C, El Agha E, Wilhelm J, Morty R, Vadász I, Mayer K, Gattenloehner S, Fink L, Matrosovich M, Li X, Seeger W, Lohmeyer J, Bellusci S, Herold S. “Influenza Virus Infects Epithelial Stem/Progenitor Cells of the Distal Lung: Impact on Fgfr2b-Driven Epithelial Repair”. PLoS Pathog. 2016 Jun; 12(6): e1005544 (IF 6.2).
Funding

Group Members
Doctoral Students
Marie Hessler, Anna-Lena Ament

