Menu
close
Ana Pardo-Saganta was born in Jaca, Spain. After receiving her Bachelor degree (BSc) in Biology from the University of Navarra (Pamplona, Spain), she joined the Department of Gene Therapy and Hepatology at the Center for Applied Medical Research (Pamplona, Spain) to carry out her PhD (2003-2008). In 2009, she joined Dr. Jayaraj Rajagopal’s laboratory at the Center for Regenerative Medicine of Massachusetts General Hospital (MGH) and Harvard Stem Cell Institute (Boston, USA) as a postdoctoral fellow (2009-2014). In 2014, she received her first faculty position as an Instructor in Medicine at Harvard Medical School and Assistant in Biology at the Division of Pulmonary and Critical Care at MGH (Boston, US) and in 2016, she joined the Department of Regenerative Medicine of CIMA Universidad de Navarra (Pamplona, Spain) as Principal Investigator after being awarded with the prestigious Ramón y Cajal Fellowship, to lead the group of “Lung stem cells and regeneration in lung disease”. Since then, all her projects have been funded by different agencies, she has published her findings in high profile journals (Nature, Cell Stem Cell) and contributed as an author to many other articles also published in top journals, and successfully mentored a number of undergraduate, master and PhD students as well as postdoctoral fellows. Noteworthy, she carries out an important task of dissemination of her research, also focused to make visible and support women in science. As a recognition of her investigation in lung regeneration and respiratory disease she was awarded by Fundación AstraZeneca (2021) and since October 2021, she is a Professor in Lung Inflammation and Repair at the Institute for Lung Health (ILH) of Justus Liebig University (JLU) in Giessen, Germany. In addition, she is a Faculty Member of the Universities of Giessen and Marburg (UGMLC), the Excellence Cluster Cardio-Pulmonary Institute (CPI) and the German Center for Lung Research (DZL).
The Pardo-Saganta lab seeks to identify the cellular and molecular mechanisms underlying lung inflammation, fibrosis and repair, focusing on the role of the complex intercellular communication between immune cells, epithelial (stem) cells and mesenchymal cells in the alveolar compartment of the adult lung in the pathogenesis, progression and resolution of disease. This knowledge will allow us to reach our major goal: to find efficient treatments for lung diseases that block the progression of the disease while promoting regeneration to recover functional tissue.
Our research investigates the interrelation between inflammation, fibrosis, regeneration and aging as relevant processes for lung homeostasis and pathogenesis. Given the complex interplay of these mechanisms and cell populations, we think that a unifying integration of all of these processes is required to understand higher order pathogenesis which can be compared to the physiology of the normal tissue. Our laboratory aims to understand the development of lung disease integrating cell interactions and the microenvironment at every step of injury progression: inflammation, fibrogenesis, resolution-repair.
Inflammation is the first response immediately triggered after injury and necessary to resolve damage. However, unresolved, uncontrolled or aberrant performance of inflammation contributes to the development of disease. Bacterial or viral infection that cause severe airway and alveolar injury, results in uncontrolled local and systemic inflammation, loss of alveolar barrier function and impairment of gas exchange, and the clinical manifestation of acute lung injury/ARDS. A tightly balanced, compartmentalized and spatially controlled immune response is therefore key to protecting the host from invading pathogens without injuring the delicate alveolar architecture and jeopardizing the vitally mandatory level of gas exchange function.
The lung epithelium is one of the major pathogen sensors and orchestrators of the initial immune response of the entire body. It elicits a multitude of cell-autonomous stress responses to cope with the invading pathogen and induces an inflammatory response by instructing circulating and tissue-resident immune cells to fight invading pathogens. This might result in rapid pathogen clearance and maintenance of parenchymal integrity at best, or in extensive tissue destruction with non-contained, systemic inflammation at worst. In fact, the epithelial surveillance mechanisms and cellular communication networks involved in the timely fine-tuning and balancing of these responses need to be tightly adjusted to the requirements of the infected and injured bronchoalveolar compartment. The mechanisms controlling this delicate balance remains unknown.
We have recently discovered a cellular interaction between airway stem cells and tissue resident immune cells necessary to maintain these antigen-presenting cells at homeostasis and that is essential to initiate an immune response. This type of intercellular communication may occur in every region of the respiratory tree in many different pathological conditions and may be a key mechanistic link between stem cell exhaustion and impaired immune response in lung disease. Thus, understanding the key role of stem cells in the regulation of the immune response will help to develop immune-mediated mechanisms of regeneration and repair that may complement existing stem cell therapies to promote functional regrowth of vital tissues.
Contrary to acute lung injury, chronic lung disease develops over extended periods of time most likely on a scenario/context of low-grade inflammation developed during aging that may predispose the tissue and contribute to the pathogenesis of age-related diseases such as IPF or COPD. In addition to dysfunctional inflammatory cells, chronic lung diseases are also characterized for failure in mechanisms of repair. In the last years, novel epithelial progenitor cells have been identified and demonstrated to contribute to lung repair. Recent findings shed light to the mechanisms of regeneration of the bronchioalveolar epithelium; however, a better understanding of the coordinated processes of tissue repair from different stem cell pools after injury is crucial to restore the delicate lung architecture. The contribution of the interplay between these epithelial progenitor populations and other cell types of their niche to the development of disease, needs further investigation (Figure 1).
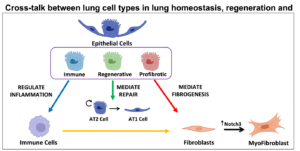

Figure 1. Overview of the cellular interplay regulating inflammation, fibrosis and regeneration addressed by the Pardo-Saganta lab
Deciphering the key cellular and molecular events balancing effective host defense and beneficial epithelial stress responses versus parenchymal damage, overshooting inflammation and aberrant repair will provide novel targets of intervention to drive injury resolution and tissue repair.
Accordingly, the professorship for Lung Inflammation and Repair aims to achieve the following objectives:
Prof. Dr. Ana Pardo-Saganta
Professor for Lung Inflammation and Repair at the Institute for Lung Health (ILH)
Cardio-Pulmonary Institute (CPI)
Member of the German Center for Lung Research (DZL)
Universities of Giessen and Marburg Lung Center (UGMLC)
Justus-Liebig University Giessen
Aulweg 132
35392 Giessen
Tel: +49 (0) 641 99 36421 (office)
Tel: +49 (0) 641 99 36413 (lab)
Email:
Ana.Pardo-Saganta@innere.med.uni-giessen.de
Web:
https://ilh-giessen.de/en/professorships/lung-inflammation-and-repair/
Dr. Sara Taghizadeh
Alejandro Egea Zorrilla
Dongzhu Li
Zurine Blasco Iturri
Marija Kupresanin
Dr. Sezin Czarnecki
Lea Frauendorfer
Deivanai Alagappan