Menu
close
István Vadász has been appointed Adjunct Faculty of the Institute for Lung Health, where he oversees the area of Hypercapnia Signaling. Dr. Vadász is deputy director of the Department of Internal Medicine and head of the Medical Intensive Care Unit at the University Hospital of the Justus Liebig University of Giessen and Adjunct Associate Professor of Medicine at the Division of Pulmonary and Critical Care Medicine at the Feinberg School of Medicine at Northwestern University in Chicago, USA. He obtained his medical degree at the University of Szeged and his PhD degree at the Justus Liebig University, followed by a postdoctoral position at the Northwestern University. Dr. Vadász is faculty member/PI of the Universities of Giessen and Marburg Lung Center (UGMLC), the German Center for Lung Research (DZL), the Excellence Cluster Cardio-Pulmonary Institute (CPI), and the DFG-funded clinical research unit Virus-induced Lung Injury (KFO309). He has published 86 research articles (September 2021) in well-renowned scientific journals, including Journal of Clinical Investigation, Proceedings of the National Academy of Science, American Journal of Respiratory and Critical Care Medicine, Intensive Care Medicine, Thorax, and Lancet Respiratory Medicine.
Dr Vadász’s lab focuses on various aspects of alveolar-capillary barrier function in the setting of acute lung injury and acute respiratory distress syndrome (ARDS) with a primary interest in alveolar epithelial transport processes that control alveolar fluid balance. The Vadász lab has also significantly contributed to the understanding of the detrimental consequences of hypercapnia (accumulation of CO2 in blood and tissue) on basic biological phenomena, including dysfunction of the alveolar epithelial barrier, the primary site of CO2 elimination. More recently, the group of Dr. Vadász has been focusing on the mechanisms of CO2 sensing in non-excitable cells of the lung and on specific hypercapnia-induced maladaptive signaling events.
Several acute and chronic lung diseases, e.g. ARDS and chronic obstructive pulmonary disease (COPD) are associated with alveolar hypoventilation leading to hypercapnia and worse outcomes. Hypercapnia, similar to hypoxia, is a fundamental gas exchange disturbance, however, in contrast to hypoxia, the biological effects of hypercapnia remain largely under-investigated. It is increasingly evident that elevated CO2 levels are deleterious, contributing to various acute and chronic pulmonary disease states. Importantly, the exact molecular mechanisms of CO2 sensing and the question, to what extent hypercapnia-driven signaling events dysregulate the alveolar-capillary barrier function as well as innate immunity and host defense and contribute to the worse outcomes observed in patients with elevated CO2 levels remain to be addressed.
The goal of the Vadász lab is the in-depth analysis of the molecular mechanisms by which elevated CO2 levels are sensed by lung cells, and of the concomitant hypercapnia-induced signaling events. While some of these signals are adaptive, the majority of the CO2-induced patterns are maladaptive. In particular, the Vadász lab aims to describe the mechanisms of hypercapnia-induced alveolar-capillary barrier dysfunction as well as the impairment of innate immunity and host defense in vitro, ex vivo and in vivo utilizing, among others, three-dimensional culture models of the distal lung, including human precision-cut lung slices (PCLS), a model of isolated, ventilated and perfused mouse lungs and various animal models of ARDS and COPD. Furthermore, by using single cell RNA sequencing, the group of Dr. Vadász proposes to identify the primary cell type responsible for CO2 sensing and the molecular sensors involved. As the CO2-induced signals partially overlap with those upon metabolic stress, the potential role of mitochondria in CO2 sensing will be investigated. Regarding the adaptive as opposed to maladaptive effects of hypercapnia on cells, tissues and organs, the role of hypercapnia on general as well as selective protein translation will be studied with a particular focus on alveolar epithelial transport processes, alveolar-capillary barrier function and host defense. Upon identification of the maladaptive signals, Dr. Vadász’s research group aims to establish novel, selective, tailored therapy options, directly delivered to the lung to specifically interfere with the CO2-induced deleterious pulmonary signals. More details as to the pathways particularly in focus are outlined below.
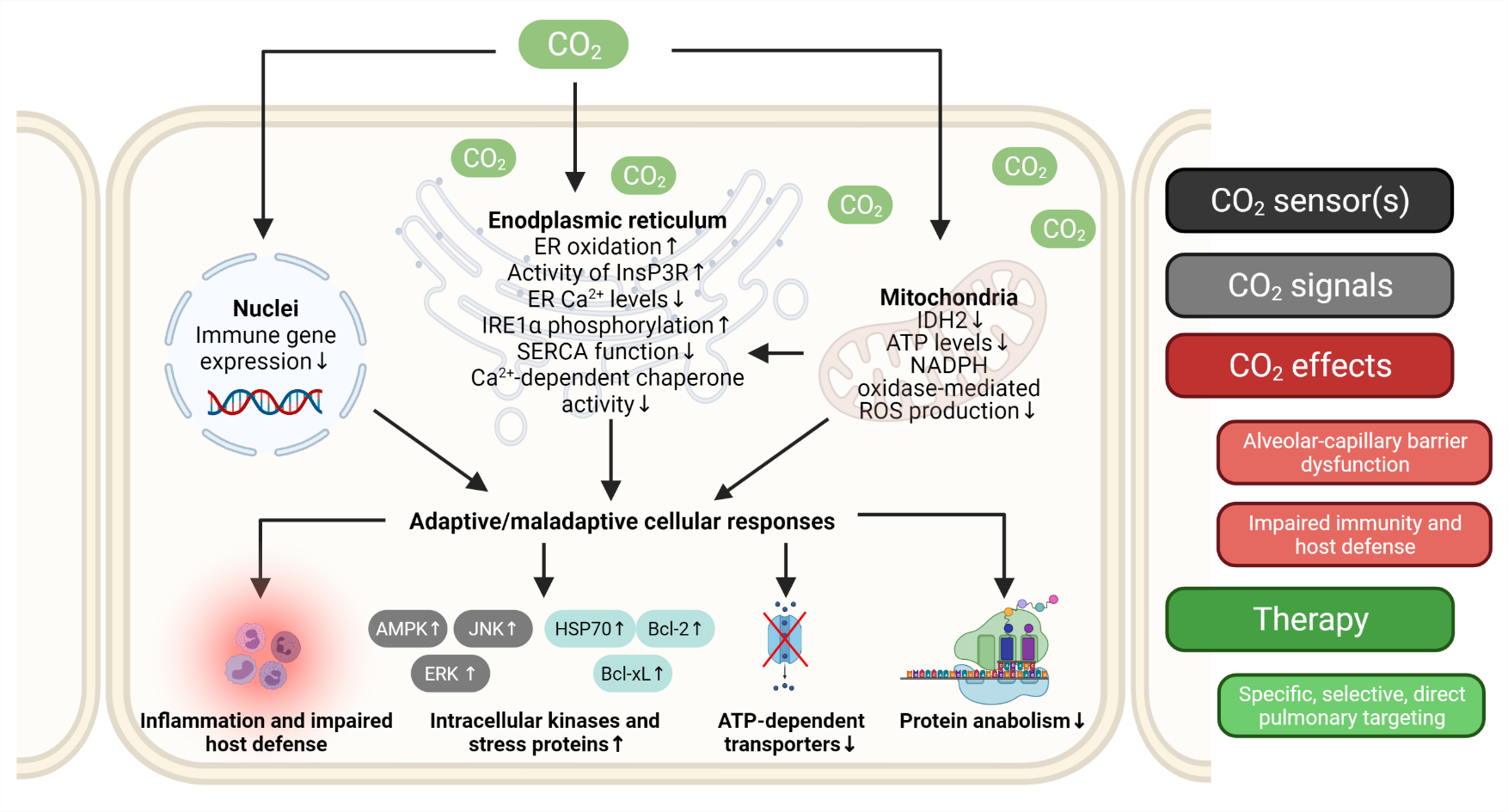
Overview of major hypercapnia-related signaling events addressed by the Vadász lab. AMPK - AMP-activated protein kinase, Bcl-xL - B-cell lymphoma-extra large protein, Bcl2 - B-cell lymphoma 2 protein, ER - endoplasmatic reticulum, ERK - extracellular signal-regulated kinase, HSP70 - heat shock protein 70, IDH2 - isocitrate dehydrogenase 2, InsP3R - ER membrane-localized inositol trisphosphate receptor; JNK - c-Jun N-terminal kinase.
Dr. Dr. István Vadász
Department of Internal Medicine
Justus Liebig University Giessen (JLU)
Universities of Giessen and Marburg Lung Center (UGMLC)
German Center for Lung Research (DZL)
The Cardio-Pulmonary Institute (CPI)
Klinikstrasse 33
D-35392 Giessen
GERMANY
Tel: +49 641 99 42358 (lab)
Fax: +49 641 985 42359
Email: istvan.vadasz@innere.med.uni-giessen.de

Group Members
Post Doc
Vitalii Kryvenko, M.D., Ph.D.
Doctoral Students
Florian Michel, M.Sc., Le-Han Nguyen, M.Sc., Johannes Pott
Technical Assistant
Miriam Wessendorf
Alumni
Dr. vet. med. Sebastian Rummel, Dr. biol. hom. Yasmin Buchäckert, Dr. rer. nat. Benno A. Grzesik, Dr. vet. med. Ramona Rühl, Nieves M. Gabrielli, Ph.D., Luciana C. Mazzocchi, Ph.D., Dr. med. Christine U. Vohwinkel, Paulina Gwoździńska, Ph.D., Andrés Alberro, M.Sc.