Menu
close
Fgf10 is a key growth factor both during lung development and in disease conditions
Fgf10 and branching morphogenesis of the embryonic lung epithelium: Fgf10 is expressed in the distal mesenchyme at sites where prospective epithelial buds will emerge [Bellusci et al., Development, 1997. 124(23): p. 4867-78]. Fgf10 null newborn mice show multiple organ defects, including lung agenesis [Sekine et al., Nat Genet, 1999. 21(1): p. 138-41]. Fgf10 is therefore a key growth factor acting on early epithelial lung progenitors. The comprehensive effect of Fgf10 signaling through Fgfr2b on these early progenitors was recently reported by our group [Jones et al. Frontiers in Genetics, 2019. 9(746)].
Fgf10 acts on the epithelium via Fgfr2b: Fgf10 is the primary ligand for Fgfr2b during early embryonic development as demonstrated by the remarkable similarity of phenotypes exhibited by embryos where these genes have been inactivated [De Moerlooze et al., Development, 2000. 127(3): p. 483-92; Ohuchi et al., Biochem Biophys Res Commun, 2000. 277(3): p. 643-9].
Fgf10 elicits a dose-response effect on the formation of the alveolar epithelial lineage. Our results indicate that Fgf10 plays an important role not only in the proliferation but also in the differentiation of the epithelial progenitor cells towards the AT2 lineage [Ramasamy et al., Dev Biol, 2007. 307(2): p. 237-47 and Chao et al. J Pathol, 2017. 241(1): p. 91-103].
Fgfr2b signaling plays a key role in the formation of the alveolar epithelial lineage as well as in the maintenance of the mature AT2s during homeostasis
The specification, characterization, and fate of alveolar type 1 and type 2 (AT1 and AT2) progenitors during embryonic lung development is poorly defined. Current models of distal epithelial lineage formation fail to capture the heterogeneity and dynamic contribution of progenitor pools present during early development. Furthermore, few studies explore the pathways involved in alveolar progenitor specification and fate.
In our ongoing project, we build upon our work at E12.5 [Jones et al., Frontiers Genetics 2019 9:746]. and at E14.5 [Jones et al. Cells 2020 9(5):1274] by investigating the role of FGFR2b signalling during late pseudoglandular/early canalicular stage (E16.5) development. We use Cre-based transgenic mouse models to conditionally inactivate FGFR2b signalling in AT1 or AT2 progenitors, and to label those cells with a tomato-RFP reporter. With these models, we are able to lineage label putative distal airway progenitors just prior to E16.5 and analyze their commitment to both AT2 and AT1 lineages in control and loss of FGFR2b signalling conditions. Our current research will help build a model for the specific role of FGFR2b signalling on distal epithelial progenitors during pseudoglandular lung development, and suggest a role for FGFR2b on a subset of a generally heterogenous population of alveolar epithelial cells during lung repair after injury.
Identification of a novel AT2 progenitor population in the lung
Our data indicate that as distal respiratory lineages emerge and develop (beginning as early as E12.5), the role of FGFR2b signalling shifts, eventually concentrating in a sub-population of the AT2 lineage. This highlights an increasingly appreciated fact that cellular populations are heterogeneous, and points to the need to identify and further classify these sub-populations of cells. Indeed, recent work from our lab has identified two major sub-populations of AT2 cells in adult mice: those expressing high levels of Sftpc and Fgfr2b, and those expressing low levels of both markers in addition to high expression of a cell surface protein called PD-L1 [Ahmadvand et al. European Respiratory Journal 2021 58(5):2004168]. This latter immature sub-population of AT2s is quiescent during homeostasis, but becomes activated during compensatory growth after pneumonectomy, eventually expanding to replenish the mature AT2 population. These cells were termed ‘injury activated alveolar progenitors’ (IAAPs), and were also found to be enriched in precision cut lung slices of human fibrotic samples [Ahmadvand et al. Cells 2022 11(10):1593], as well as in the context of Fgfr2b deletion in SFTPCPos cells during homeostasis in the mouse [Ahmadvand et al. Cell Mol Life Sci 2022 79(6):302]. The engagement of this quiescent population in response to injury likely depends on FGFR2b signalling
Identification of Fgf10Pos cells using lineage tracing. We have previously generated a Fgf10Cre-ERT2 knock-in mouse line that allows lineage tracing of Fgf10Pos cells during development and postnatally [El Agha et al., PLoS One, 2012. 7(6): p. e38452]. Using these mice, we demonstrated the presence of two waves of Fgf10Pos cells during embryonic lung development [El Agha et al., Development, 2014. 141(2): p. 296-306]. One of the limitations of this Fgf10CreERT2 line is that the insertion of the CreERT2 cassette into the first exon, in frame with the ATG, also led to the deletion of important transcription factor binding sites in the intron 1. This led to the loss of expression of the CreERT2 after birth. To circumvent this limitation, we have generated a new Fgf10CreERT2 line with the insertion of the CreERT2 cassette in within the 5’UTR of the Fgf10 gene [Chu et al. Frontiers in Cell and Developmental Biology 2021 9:671841 ].
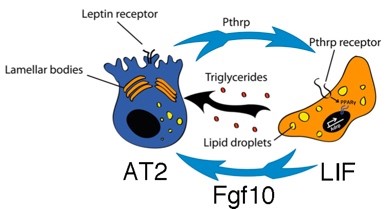
Fgf10 controls lipofibroblast (LIF) differentiation during embryonic lung development. Lipid-containing alveolar interstitial fibroblasts (lipofibroblasts or LIFs) are recognized as a crucial component of the AT2 stem cell niche in the rodent lung. Although LIFs were initially believed merely to assist AT2 cells in surfactant production during neonatal life, recent evidence suggests that these cells are indispensable for survival and growth of epithelial stem cells during adulthood. Despite increasing interest in LIF biology, little is known about their cellular origin or the molecular pathways controlling their formation during embryonic development. We have shown that a population of lipid-droplet-containing stromal cells emerges in the developing mouse lung between E15.5 and E16.5 [Al Alam et al., Development, 2015. 142(23): p. 4139-50]. In vivo knockdown of Fgfr2b ligand activity and reduction in Fgf10 expression leads to global reduction in the expression levels of LIF markers at E18.5. We also demonstrated using human fetal lungs the relevance of our discoveries to humans [Al Alam et al., Development, 2015. 142(23): p. 4139-50]. Our results revealed an essential role for Fgf10 signaling in LIF differentiation.
LIFs give rise to activated myofibroblasts (MYFs) during lung fibrosis. We recently reported, using in vivo lineage tracing tools in the context of fibrosis induction and resolution (bleomycin model) that LIFs transdifferentiate into activated MYFs and that a significant proportion of the labeled activated MYFs transdifferentiate into LIFs during fibrosis resolution [El Agha et al., Cell Stem Cell, 2017. 20(2): p. 261-273 e3]. Interestingly, fibrosis in the lung is thought to originate from repetitive AT2 injury. Loss of AT2 cells may lead to the transdifferentiation of the LIFs into activated MYFs. We have also written a comprehensive review on the role of mesenchymal stem cells in fibrosis formation [El Agha et al., Cell Stem Cell, 2017. 21(2): p. 166-177].
Lung stromal cells (PdgfraPos) are instrumental for AT2 cells growth in vitro (alveolospheres). Lineage-labeled AT2 cells isolated by FACS, were mixed with primary PdgfrαPos lung stromal cells and placed into 3D culture (in growth factor reduced Matrigel). Under such conditions, these AT2 cells gave rise to self-renewing "alveolospheres," which contained both AT2s and cells expressing multiple AT1 markers [Barkauskas et al., J Clin Invest, 2013. 123(7): p. 3025-36]. This stromal population included LIFs that are expressing Adipose related protein (Adrp) and Pdgfra [Ntokou et al., Am J Physiol Lung Cell Mol Physiol, 2015. 309(9): p. L942-58]. LIFs normally reside close to AT2s. LIF may therefore constitute a stem cell niche for AT2 cells in the murine lung. Similar dynamic exists between AT2 and mesenchymal cells in the human lung. More recently, the Morrisey lab showed that AT2 supportive cells, called mesenchymal alveolar niche cells (MANC) are co-expressing Axin2, Pdgfra and Fgf7 [Zepp et al., Cell, 2017. 170(6): p. 1134-1148 e10]. These cells are likely different from the PdgfraPos Fgf10Pos LIFs. Using alveolosphere assays to determine the activity of the mesenchymal niche for AT2 cells, we have identified the resident mesenchymal cells expressing Sca1 and Fgf10 to be a priviledge niche for AT2 stem cells [Taghizadeh et al. Stem Cells 2021 39(10):1382-1394].
Fgf10 and lung disease. Fgf10 is also a major player in the regeneration of the lung after injury. In the mouse model of bleomycin-induced lung fibrosis, Fgf10 overexpression during fibrosis formation or fibrosis resolution demonstrated a protective and therapeutic effect, respectively [Gupte et al., Am J Respir Crit Care Med, 2009. 180(5): p. 424-36]. Fgf10 is also involved in the regeneration of the bronchial lung epithelium after naphthalene injury [Volckaert et al., J Clin Invest, 2011. 121(11): p. 4409-19 and Moiseenko et al. Cell Reports 2020. 33(12);108549]. Patients with heterozygous loss of function of FGF10 exhibit a significant decrease in IVC, FEV1 and FEV1/IVC quota compared to non-carrier siblings and predicted reference values [Klar et al., J Med Genet, 2011. 48(10): p. 705-9], consistent with chronic obstructive pulmonary disease. In human, exposure to inflammation increases the risk for developing broncho-pulmonary dysplasia (BPD) [Klinger et al. Pediatrics, 2010. 125(4): p. e736-40]. Interactions between NF-kB, Sp1 and Sp3 led to inhibition of Fgf10 expression [Carver et al. J Biol Chem, 2013. 288(21): p. 15318-25]. Fgf10 inhibition is mediated by toll-like receptor 2 and 4 (Tlr2 or Tlr4) activation and decreased FGF10 concentration was found in lung samples from children suffering from BPD [Benjamin et al. Am J Physiol Lung Cell Mol Physiol, 2007. 292(2): p. L550-8]. We also published that an Fgf10-Hippo epithelial mesenchymal crosstalk maintains and recruits lung basal stem cells in the conducting airways [Volckaert et al. Dev Cell, 2017. 43(1): p. 48-59 e5]. While transient Fgf10 expression by ASMCs is critical for proper airway epithelial regeneration in response to injury, sustained Fgf10 secretion by the ASMC niche, in response to chronic Ilk/Hippo inactivation, results in pathological changes in airway architecture. We also identified a new population of repair supportive mesenchymal cells (RSMCs), distinct from the ASMCs but which also secrete Fgf10 for the repair of the bronchial epithelium [Moiseenko et al. Cell Reports 2020. 33(12);108549]. Therefore, Fgf10/Fgfr2b signaling is an interesting target to treat lung diseases.
Relevance of the AT2 cells for lung homeostasis and disease. Our main research interest focusses on the role of FGF10 on AT2 cells. AT2s are crucial for lung homeostasis as they are acting as stem cells (self-renewal and can differentiate in AT1 cells). These cells also make the surfactant, which is essential for lung function. Finally, these cells are the prime target for cancer and virus infection including SARS-Cov-2 causing the current pandemic [Desai et al. Nature, 2014. 507(7491): p. 190-4].
Overall, Fgf10 is a crucial regulator of 1) LIF-AT2 interactions during homeostasis and in aging condition and 2) is potentially capable of stimulating the formation of new alveoli after injury. Our long-term goal is to design FGF10-based therapies to enhance repair after injury.
 Our main goals are:
Our main goals are:
1) to characterize AT2 and LIF heterogeneity in the adult lung using lineage tracing approaches and
2) to investigate, during homeostasis and repair after injury, the role of Fgf10 signaling, via Fgfr2b both in AT2 and in LIF, using cell autonomous and non-cell autonomous based approaches.
3) to optimize the use of recombinant FGF10 for translational applications in human.
Cooperative Projects in the ILH
Prof. Dr. Saverio Bellusci
Stem cells and Extracellular Lung matrix remodelling
ECCPS, Justus Liebig University Giessen
Aulweg 130
35392 Giessen
Tel: +49 (0) 641 99 46730
Fax: +49 (0) 641 99 46739
Email: saverio.bellusci@innere.med.uni-giessen.de
Post Docs: Stefano Rivetti, Manuela Maregua, Xuran Chu
Doctoral Students: Esmeralda Vasquez Pacheco, Leila Sotoodeh Atefi, Afshin Noori, Arun Lingampally
Technical Assistants: Kerstin Goth