Overview of the relevance of mitochondria in lung diseases
In lung diseases, mitochondria have gained attention due to their role as a central hub for integration and transformation of cellular signals, thereby regulating, for example, inflammation, proliferation, apoptosis, and contraction. In addition, mitochondria act as primary sensors for hypoxia and cellular metabolites. Mitochondrial physiology and biochemistry thus go far beyond the central function of these organelles in the production of ATP by means of oxidative phosphorylation. However, the complex interactions of various mitochondrial functions—such as oxidative phosphorylation, regulation of cellular redox and calcium homeostasis, and adaptation of mitochondrial morphology—represent a major challenge for the analysis and pharmacological targeting of mitochondria.
In the lungs, the release of mitochondrial reactive oxygen species (ROS), regulation of the intracellular and sarco-/endoplasmic calcium concentration, and the modulation of metabolites collectively play an important role in the development of various pathologies. These include pulmonary hypertension (PH) and pulmonary emphysema. In addition, mitochondria affect physiological signaling processes—for instance, hypoxia-dependent signaling pathways or the activation of immune cells. Although a large number of animal models have shown the effectiveness of targeting mitochondria for the treatment of various pulmonary diseases, translation into clinical practice has been very limited. Therefore, the aim of the research of the Sommer group is to gain a deeper understanding of physiological and pathological mitochondrial signal processes in the lung that allow the development of new treatment strategies and the implementation of experimental concepts in clinical practice.
Research areas of the Sommer group
As a clinician scientist with a strong background in basic science, Prof. Sommer focusses on the transfer of fundamental, preclinical scientific concepts to clinical practice, as well as the implementation of clinical questions into experimental problem solutions ("from bench to bedside and from bedside to bench"). This has been achieved by setting up a basic scientific working group in the field of pulmonary vascular and airway remodeling with a focus on mechanisms dependent on mitochondria and ROS. In parallel, clinical work guarantees close contact to patients and other clinicians for optimal translational research and conduct of clinical trials.
Specific research areas:
We identified a fundamental, acute oxygen-sensing mechanism of mitochondria not only in the pulmonary vessels but also in the carotid body against the background of a very long historical debate about sensory and signaling mechanisms in acute hypoxia. This discovery is the basis for the development of therapeutic approaches for ventilation perfusion mismatch, such as those occurring in sepsis and COPD (Moreno-Dominguez et al., Sci Signal. 2020 Jan 21;13(615):eaay9452; Sommer et al., Sci Adv. 2020 Apr 15;6(16):eaba0694; Sommer et al., Circ Res. 2017;121(4):424-438; Gierhardt et al., Eur Respir Rev. 2021 Sep 15;30(161):210059).
The inhibition of peroxynitrite formation in cigarette smoke-induced pulmonary emphysema was established as a new therapeutic approach. This can be achieved by inhibiting the inducible NO synthase (iNOS) or the NADPH oxidase subunit NoxO1. These studies offer new starting points for the development of clinical therapies, such as via iNOS inhibitors (Seimetz, Sommer et al., Nat Metab. 2020 Jul;2(7):648). Furthermore, the soluble guanylate cyclase was identified as potential therapeutic target in smoke-induced pulmonary emphysema and PH (Pichl, Sommer et al., Eur Respir J. 2019 Jun 27;53(6):1802445). Further studies aim to decipher mitochondrial mechanisms in the development of smoke-induced pulmonary emphysema, especially with regard to the release of mitochondrial ROS, which leads to a) hyperinflammation, chronic bronchitis, and exacerbations; b) lung destruction and the development of emphysema; c) proliferative mechanisms and PH; and d) an impaired adaptive immune response.
The role of ROS and changes in mitochondrial physiology in the development of hypoxia-dependent and hypoxia-independent forms of PH has been successfully elucidated (Gierhardt et al., Cardiovasc Res. 2022 Jan 7;118(1):305-315; Pak et al., Am J Respir Cell Mol Biol. 2021 Aug;65(2):226-228; Pak et al., Eur Respir J. 2018. pii: 1701024; Pak et al., J Respir Cell Mol Biol. 2013 Sep;49(3):358-67.). Various mitochondrial ROS modulators (MitoQ, S3QEL, MitoTempo), as well as genetic models for modulating ROS (e.g., cell-type-specific Cox4i2 or UCP2 knockout mice) are available to determine the role of ROS in the development of PH and to investigate non-ROS related mechanisms in PH.
Specific methodology:
1) Mitochondrial methods: Respirometry (also in combination with fluorescence measurements), ROS detection using fluorescent dyes/ protein-based probes/electron spin resonance spectroscopy, redox measurements using remission spectrophotometry, determination of mitochondrial morphology and calcium regulation by confocal microscopy and 3D live cell imaging, as well as biochemical methods, for example, Blue Native Gels and Complexome analyses.
2) Animal models: Murine smoke-induced pulmonary hypertension, monocrotaline-induced pulmonary hypertension, murine smoke-induced pulmonary emphysema and hypertension, double-hit model of smoke-induced emphysema and virus infection, hyperoxia-induced pulmonary damage, LPS-induced acute lung injury, ex vivo isolated ventilated and perfused mouse lung.
2) Clinical studies/samples: Human material, such as serum samples, peripheral blood mononuclear cells, bronchoalveolar lavage (BAL), biopsies, and cultured lung sections are available from patients with COPD and PH.
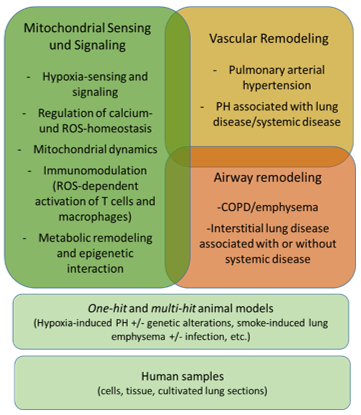
Figure 1: Overview of research interests of the Sommer group
Cooperative Projects in the ILH
Prof. Dr. med. Natascha Sommer
Professorship for "Mitochondrial signalling in pulmonary vascular and airway diseases"
Senior Physician Dept. of Pneumology, Medical Clinic II
Excellencecluster Cardio-Pulmonary Institute (CPI)
Universities of Giessen and Marburg Lung Center (UGMLC)
Member of the German Lung Center (DZL)
Justus-Liebig-University Giessen
Aulweg 130
35392 Giessen
Tel: +49 641 99 42471 (Direct)
Tel: +49 641 99 42422 (Assistant)
Tel: +49 641 985 57030 (Clinic)
Email: Natascha.Sommer@innere.med.uni-giessen.de