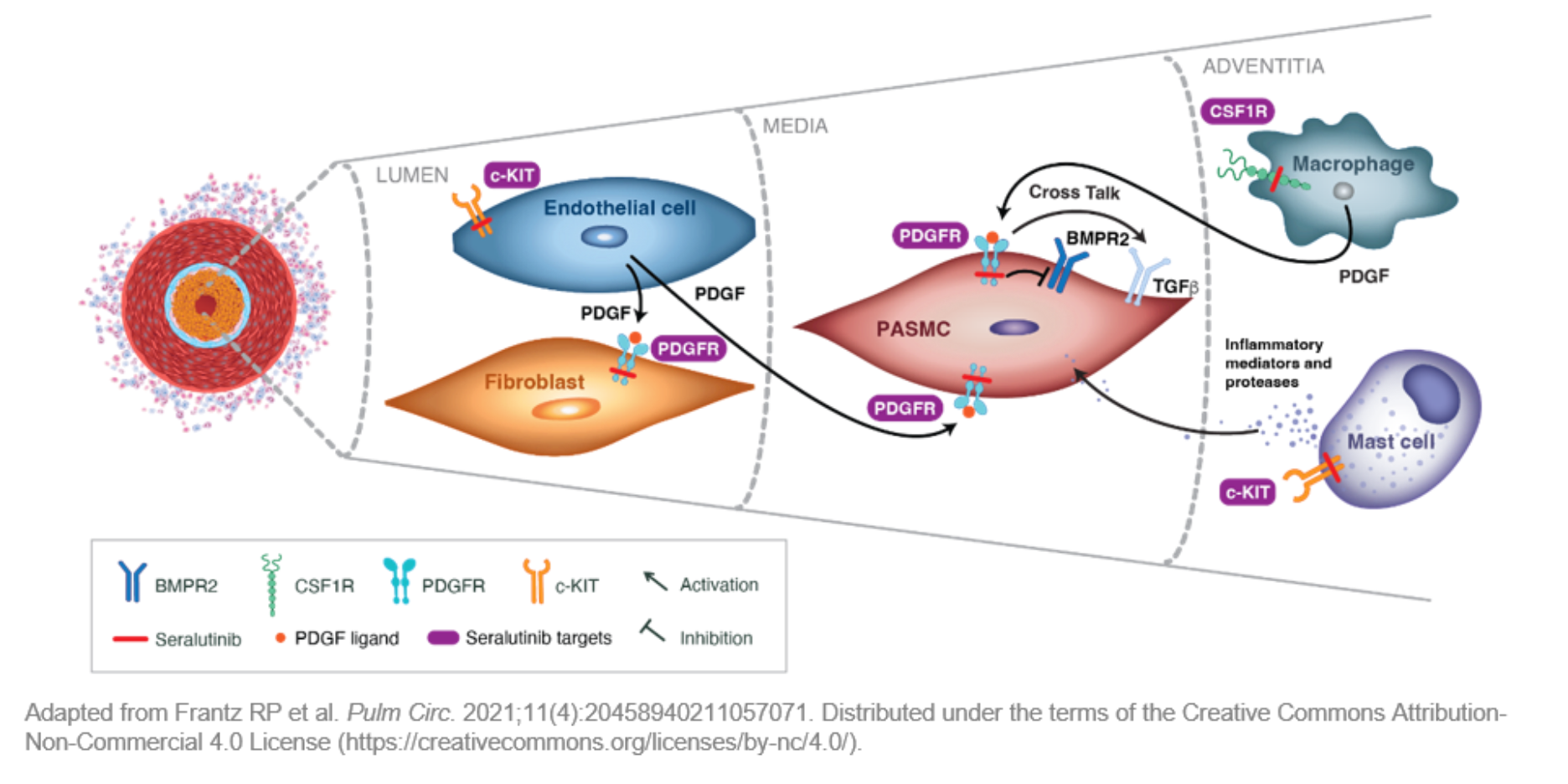
Morbidity and mortality in pulmonary arterial hypertension (PAH) remain high due to inflammatory, proliferative, and fibrotic pathways driven by kinase activation. Seralutinib, an inhaled kinase inhibitor, targets these pathways. This study evaluated the efficacy and safety of seralutinib in PAH patients on standard therapy. The TORREY trial was a phase 2, double-blind, placebo-controlled study involving 86 PAH patients randomized to receive seralutinib or placebo for 24 weeks. The primary endpoint was change in pulmonary vascular resistance (PVR) at 24 weeks. Seralutinib significantly decreased PVR compared to placebo, with a mean difference of -96.1 dyne·s/cm5. The most common adverse event was cough. Thus, inhaled seralutinib effectively reduced PVR in PAH patients on background therapy. Built upon the positive results of the TORREY study currently a pivotal phase III randomized controlled study, the PROSERA study is underway.
Find the full article here:
Source: June 2024 (cpi-online.de)