GoDeep - PVRI Global Deep Phenotyping PH Registry
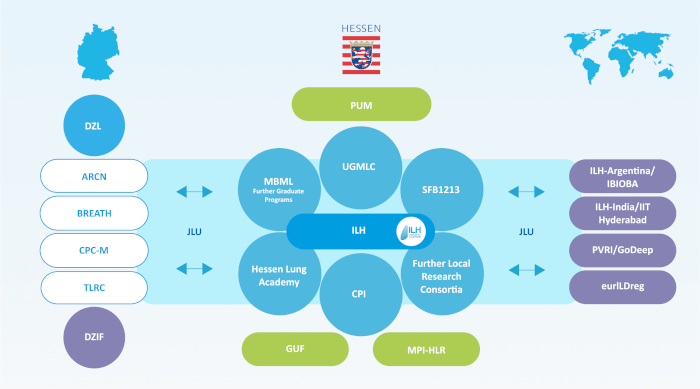
GoDeep is a deep phenotyping pulmonary hypertension (PH) meta-registry, integrating the data acquired in all PH disease groups from PH referral centers worldwide and spanning all continents. GoDeep is an initiative that was co-founded by the Pulmonary Vascular Institute (PVRI) and the Justus Liebig University Giessen in Giessen, Germany; and is overseen as a project of the Institute for Lung Health (ILH) in Giessen, Germany. Data are acquired from participating centers using a parameter framework that has been agreed upon by all centers, and anonymized data are transferred from the respective centers to a central coordination office at the ILH, in line with guidelines established by the relevant regulatory authorities.
The GoDeep registry provides very rich datasets that allow for the evaluation of a multitude of epidemiological and scientific questions, which demands an appreciable number of enrollees, that suitably represent the broad geographical and ethnic spectrum of PH. A highly qualified team of statisticians and information technologists ensures the necessary quality control processes and statistical evaluation of the data sets.
Key Features as of date
| Number of PH patients | > 35,000 |
| Comparator Subjects without PH | > 3,000 |
| Data Points | > 8,000,000 |
| Number of parameters/variables | 350 |
| PH diagnosis class WSPH 2013/2018 and/or WSPH 2024 | ✓ |
| WHO functional class PH | ✓ |
| Comorbidities | ✓ |
| Survival & cause of death | ✓ |
| Right heart catheterization (mandatory for registry entry) | ✓ |
| Spiroergometry | ✓ |
| Echocardiography | ✓ |
| 6-minute walk test | ✓ |
| Lung function test | ✓ |
| PH specific drug therapy | ✓ |
| Imaging methods MRI / Chest CT | ✓ |
| Availability of bio samples | ✓ |
Distribution of PH groups of the entire current GoDeep cohort
Distribution of mPAP of the entire current GoDeep cohort
Phenotyping parameters
Our database contains up to 350 different parameters per patient consultation/visit. All parameters are annotated with international standard terminology codes (such as those provided by the Logical Observation Identifiers Names and Codes, LOINC, and the Systematized Nomenclature of Medicine-Clinical Terms, SNOMED-CT, databases). A detailed list of parameters is provided below.
Data protection
Data processing conforms to the current standard of General Data Protection Regulation of the European Union. Prior to transfer to the PVRI GoDeep registry, all data are anonymized using k-anonymization, which is overseen by local the data protection officers affiliated with each center.
Database and analysis tools
The storage of all observational data conforms to the OMOP common data model (CDM). For data analyses, complex algorithms and statistical calculations can be run either directly on the OMOP CDM or on data extracts that are supplied by the GoDeep team. Additionally, simple analysis tools are available via web browser to all members.
List of parameters: these include mandatory, essential, recommended and extended parameter sets:
| Ebene 1 | Ebene 2 | Ebene 3 | Ebene 4 |
|---|---|---|---|
| Personal data | Birth decade | ||
| Personal data | Sex | ||
| Personal data | Ethnic group | Caucasian | |
| Personal data | Ethnic group | Hispanic | |
| Personal data | Ethnic group | Black | |
| Personal data | Ethnic group | Asian | |
| Personal data | Ethnic group | Other | |
| Personal data | Deceased number of months after diagnosis | ||
| Personal data | Survival status | alive | |
| Personal data | Survival status | deceased | |
| Personal data | Cause of death | Heart failure | |
| Personal data | Cause of death | Malignant disease | |
| Personal data | Cause of death | Renal failure | |
| Personal data | Cause of death | Pulmonary infect | |
| Personal data | Cause of death | Right heart decompensation | |
| Personal data | Cause of death | Respiratory insufficiency | |
| Personal data | Cause of death | Sepsis/MODS/SIRS/multi organ failure | |
| Personal data | Cause of death | Transplantation | |
| Personal data | PH Family History | ||
| Personal data | Transplantation Date | ||
| Diagnosis | Age at diagnosis | ||
| Diagnosis | Date of diagnosis (decade) | ||
| Diagnosis | Date of onset of PH self-reported months before diagnosis | ||
| Diagnosis | Date of onset of underlying disease prior to development of PH | ||
| Diagnosis | PH diagnosis class (WS2013 Nice, WS2008 Dana Point) | ||
| Diagnosis | Comorbidities | Coronary heart disease | |
| Diagnosis | Comorbidities | Arterial hypertension | |
| Diagnosis | Comorbidities | Venous thromboembolism | |
| Diagnosis | Comorbidities | Diabetis mellitus | |
| Diagnosis | Comorbidities | Thyroid disease | |
| Diagnosis | Comorbidities | Obstructive sleep apnoea | |
| Diagnosis | Comorbidities | Atrial fibrillation | |
| Diagnosis | Comorbidities | Parenchymal lung disease / Interstitial Lung Disease | |
| Diagnosis | Comorbidities | COPD | |
| Diagnosis | Comorbidities | Asthma | |
| Diagnosis | Comorbidities | COVID 19 | |
| Follow-up | Visit in number of months since diagnosis | ||
| Follow-up | WHO functional class (also NYHA) | ||
| Follow-up | Body weight | ||
| Follow-up | Body height | ||
| Follow-up | BMI (calculated) | ||
| Follow-up | Body surface area (BSI, calculated) | ||
| Follow-up | Smoking status | Active smoker | |
| Follow-up | Smoking status | Ex-smoker | |
| Follow-up | Smoking status | Never-smoker | |
| Follow-up | Smoking status | Packyears | |
| Follow-up | Visit reason | Hospitalization due to worsening of PH | |
| Follow-up | Visit reason | Regular / planned viist | |
| Right heart catheterization (follow up) | Date in number of months since diagnosis | ||
| Right heart catheterization (follow up) | mPAP | ||
| Right heart catheterization (follow up) | sPAP | ||
| Right heart catheterization (follow up) | dPAP | ||
| Right heart catheterization (follow up) | mSAP | ||
| Right heart catheterization (follow up) | dSAP | ||
| Right heart catheterization (follow up) | sSAP | ||
| Right heart catheterization (follow up) | Heart rate | ||
| Right heart catheterization (follow up) | PAWP | ||
| Right heart catheterization (follow up) | LVEDP | ||
| Right heart catheterization (follow up) | CVP | ||
| Right heart catheterization (follow up) | SVR | ||
| Right heart catheterization (follow up) | PVR | PVR by Fick method | |
| Right heart catheterization (follow up) | PVR | PVR by thermo dilution | |
| Right heart catheterization (follow up) | CI | CI by Fick method | |
| Right heart catheterization (follow up) | CI | CI by thermo dilution | |
| Right heart catheterization (follow up) | CO | CO by Fick | |
| Right heart catheterization (follow up) | CO | CO by thermo dilution | |
| Right heart catheterization (follow up) | PVRI | PVR Index by Fick | |
| Right heart catheterization (follow up) | PVRI | PVR Index by thermo dilution | |
| Right heart catheterization (follow up) | Pulmonary artery blood SO2 | ||
| Right heart catheterization (follow up) | Oxygen saturation | ||
| Right heart catheterization (follow up) | Peripheral saturation | ||
| Spiroergometry | Date in number of months since diagnosis | ||
| Spiroergometry | VO2 max/kg | ||
| Spiroergometry | min EQCO2 | ||
| Spiroergometry | Slope VE/VCO2 | ||
| Spiroergometry | Maximum oxygen pulse | ||
| Spiroergometry | PETCO2 | ||
| Spiroergometry | Heart Rate peak | ||
| Spiroergometry | VO2 peak | ||
| Spiroergometry | VO2 peak %pred | ||
| Spiroergometry | VO2 AT | ||
| Spiroergometry | VCO2 peak | ||
| Spiroergometry | VE peak | ||
| Spiroergometry | VE AT | ||
| Spiroergometry | MBB | ||
| Spiroergometry | Systolic BP peak | ||
| Spiroergometry | Diastolic BP peak | ||
| Spiroergometry | SPO2 at rest | ||
| Spiroergometry | SpO2 peak | ||
| Echocardiography | Date in number of months since diagnosis | ||
| Echocardiography | RA area in cm2 | ||
| Echocardiography | RA dilation | ||
| Echocardiography | LA area in cm2 | ||
| Echocardiography | LA dilation | ||
| Echocardiography | TAPSE in mm | ||
| Echocardiography | TDI velocity (S prime/dash) | ||
| Echocardiography | sPAP in mm incl CVP | ||
| Echocardiography | CVP | ||
| Echocardiography | ESA | ||
| Echocardiography | EDA | ||
| Echocardiography | FAC% of total area of right ventricle | ||
| Echocardiography | Pericardial effusion | ||
| Echocardiography | Tricuspid Regurgitation | ||
| Echocardiography | Mitral Regurgitation | ||
| Echocardiography | Aortic Regurgitation | ||
| Echocardiography | Pulmonic Regurgitation | ||
| Electrocardiography | Date in number of months since diagnosis | ||
| Electrocardiography | Sinus rhyth normal | ||
| Electrocardiography | Cardiac arrhythmia | Atrial arrhythmia | Atrial fibrillation |
| Electrocardiography | Cardiac arrhythmia | Atrial arrhythmia | Atrial flutter |
| Electrocardiography | Cardiac arrhythmia | Ventricular arrhythmia | |
| Electrocardiography | RV strain | ||
| Electrocardiography | Right atrial enlargement | ||
| Electrocardiography | Right bundle branch block (RBBB) | ||
| 6 minute walk test | Date in number of months since diagnosis | ||
| 6 minute walk test | Distance walked | In meters | |
| 6 minute walk test | Distance walked | In feet | |
| 6 minute walk test | Exercise prematurely interrupted | Minutes walked before interruption | |
| 6 minute walk test | Oxygen saturation at end of 6mwt | ||
| 6 minute walk test | Oxygen saturation at rest / before 6mwt | ||
| 6 minute walk test | Supplemental oxygen L/min | ||
| 6 minute walk test | Borg dyspnea scale at rest / before 6MWT | ||
| 6 minute walk test | Borg dyspnea scale at end of 6MWT | ||
| Laboratory tests | Date in number of months since diagnosis | ||
| Laboratory tests | BNP | ||
| Laboratory tests | NT-proBNP | ||
| Laboratory tests | GGt | ||
| Laboratory tests | Uric acid | ||
| Laboratory tests | Hemoglobin | ||
| Laboratory tests | Iron | ||
| Laboratory tests | CRP | ||
| Laboratory tests | Creatinine | ||
| Laboratory tests | ALT | ||
| Laboratory tests | AST | ||
| Laboratory tests | Total Bilirubin | ||
| Laboratory tests | Sodium | ||
| Laboratory tests | Total protein | ||
| Laboratory tests | Albumin | ||
| Laboratory tests | INR | ||
| Laboratory tests | WBC | ||
| Laboratory tests | Platelets | ||
| Laboratory tests | Alkaline phosphatase | ||
| Laboratory tests | Estimated glomerular filtration rate | ||
| Blood gas analysis (BGA) | Date in number of months since diagnosis | ||
| Blood gas analysis (BGA) | paO2 | ||
| Blood gas analysis (BGA) | paCO2 | ||
| Blood gas analysis (BGA) | Supplementary oxygen (yes/no) | Supplementary oxygen in l/min | |
| MRI imaging measurements | Date in number of months since diagnosis | ||
| MRI imaging measurements | Heart rate | ||
| MRI imaging measurements | RVEDV | ||
| MRI imaging measurements | RVESV | ||
| MRI imaging measurements | RV mass | ||
| MRI imaging measurements | RV EF | ||
| MRI imaging measurements | LVEDV | ||
| MRI imaging measurements | LVESV | ||
| MRI imaging measurements | LV mass | ||
| MRI imaging measurements | LVEV | ||
| Chest CT | |||
| V/Q scan | |||
| Quality of Life | Date in number of months since diagnosis | ||
| Quality of Life | SF36 Questionnaire | ||
| Quality of Life | emPHasis-10 Questionnaire | Frustrated by breathlessness | |
| Quality of Life | emPHasis-10 Questionnaire | Breathlessness interrupts conversations | |
| Quality of Life | emPHasis-10 Questionnaire | PH controls life | |
| Quality of Life | emPHasis-10 Questionnaire | Need rest during day | |
| Quality of Life | emPHasis-10 Questionnaire | Feeling exhausted | |
| Quality of Life | emPHasis-10 Questionnaire | Having no energy | |
| Quality of Life | emPHasis-10 Questionnaire | Breathless after on flight of stairs | |
| Quality of Life | emPHasis-10 Questionnaire | Confidence in public places | |
| Quality of Life | emPHasis-10 Questionnaire | Being dependent | |
| Quality of Life | emPHasis-10 Questionnaire | Feeling like a burden | |
| Quality of Life | SGRQ Saint Georges Respiratory Questionnaire | ||
| Quality of Life | UCSD - SOBQ (Universal Shortness Of Breath Q) | ||
| Quality of Life | PGIS (Physicians Global Impression for Symptoms) | ||
| Quality of Life | PGIC Physicians Global Impression for Change | ||
| Quality of Life | PROMIS Questionnaire for Physical Function | ||
| Lung function | Date in number of months since diagnosis | ||
| Lung function | FEV1 | Absolute value | |
| Lung function | FEV2 | Predicted value | |
| Lung function | FEV3 | %predicted value | |
| Lung function | FVC | Absolute value | |
| Lung function | FVC | Predicted value | |
| Lung function | FVC | %predicted value | |
| Lung function | TLC | Absolute value | |
| Lung function | TLC | Predicted value | |
| Lung function | TLC | %predicted value | |
| Lung function | RV | Absolute value | |
| Lung function | RV | Predicted value | |
| Lung function | RV | %predicted value | |
| Lung function | DLCO/VA | Absolute value | |
| Lung function | DLCO/VA | Predicted value | |
| Lung function | DLCO/VA | %predicted value | |
| Lung function | DLCO adj Hgb | ||
| Lung function | DLCO | Absolute value | |
| Lung function | DLCO | Predicted value | |
| Lung function | DLCO | %predicted value | |
| Lung function | VA | Absolute value | |
| Lung function | VA | Predicted value | |
| Lung function | VA | %predicted value | |
| Lung function | VC | Absolute value | |
| Lung function | VC | Predicted value | |
| Lung function | VC | %predicted value | |
| Lung function | FEV1/FVC | Absolute value | |
| Lung function | FEV1/FVC | Predicted value | |
| Lung function | FEV1/FVC | %predicted value | |
| Schistosomiasis Liver Ultrasound | Date in number of months since diagnosis | ||
| Schistosomiasis Liver Ultrasound | Does the test show periportal fibrosis | ||
| Schistosomiasis Liver Ultrasound | Does the test show enlargement of the left lobe or liver | ||
| Schistosomiasis Liver Ultrasound | Summary of additional results | ||
| Schistosomiasis Diagnosis | Has schistosomiasis ever been diagnosed | ||
| Schistosomiasis Diagnosis | Appoximate age of first infection | Unknown | |
| Schistosomiasis Diagnosis | Appoximate age of first infection | As a child (<13 yo) | |
| Schistosomiasis Diagnosis | Appoximate age of first infection | As a teenager (13-19 yo) | |
| Schistosomiasis Diagnosis | Appoximate age of first infection | As an adult (>19 yo) | |
| Schistosomiasis Diagnosis | Appoximate age of first infection | Specify the approximate date | |
| Schistosomiasis Diagnosis | Date of first infection in months after/before PH diagnosis (if known) | ||
| Schistosomiasis Diagnosis | Is there a positive test for schistosomiasis | ||
| Schistosomiasis Diagnosis | Approximate age at the first positive test | ||
| Schistosomiasis Diagnosis | Test method of diagnosis | Unknown | |
| Schistosomiasis Diagnosis | Test method of diagnosis | Kato-katz test | |
| Schistosomiasis Diagnosis | Test method of diagnosis | Rectal biopsy | |
| Schistosomiasis Diagnosis | Test method of diagnosis | Rectal biopsy | |
| Schistosomiasis Diagnosis | Test method of diagnosis | Urine CCA study | |
| Schistosomiasis Diagnosis | Location where schistosomiasis exposure may have occurred | ||
| Schistosomiasis Diagnosis | Exposure duration in years | ||
| Schistosomiasis Diagnosis | Were there ever been a diagnosis associated with portal hypertension | Date of portal hypertension associated diagnosis | |
| Schistosomiasis Diagnosis | Activities associated with schistosomiasis | Fishing | |
| Schistosomiasis Diagnosis | Activities associated with schistosomiasis | Washing | |
| Schistosomiasis Diagnosis | Activities associated with schistosomiasis | Swimming | |
| Schistosomiasis Diagnosis | Activities associated with schistosomiasis | Other activities | |
| Schistosomiasis Diagnosis | Treatment | Was there any treatment for schistosomiasis? | |
| Schistosomiasis Diagnosis | Treatment | What was the first drug to treat schistosomiasis? | |
| Schistosomiasis Diagnosis | Treatment | Approximate age at the time of treatment | As a child (<13 yo) |
| Schistosomiasis Diagnosis | Treatment | Approximate age at the time of treatment | As a teenager (13-19 yo) |
| Schistosomiasis Diagnosis | Treatment | Approximate age at the time of treatment | As an adult (>19 yo) |
| Schistosomiasis Diagnosis | Treatment | Approximate age at the time of treatment | Specify the approximate date |
| Schistosomiasis Diagnosis | Treatment | Date when treatment began (if known) | |
| Schistosomiasis Diagnosis | Conditions associated with schistosomiasis | Esophageal varicies | Date of diagnosis of esophageal varicies (if known) |
| Schistosomiasis Diagnosis | Conditions associated with schistosomiasis | Splenomegaly | Date of diagnosis of splenomegaly (if known) |
| Schistosomiasis Diagnosis | Conditions associated with schistosomiasis | Dilation of the portal vein | Date of diagnosis of dilation of the portal vein (if known) |
| Genetic Analysis | Date in number of months since diagnosis | ||
| Genetic Analysis | Mutation status | BMPR2 | |
| Genetic Analysis | Mutation status | ACVRL1 | |
| Genetic Analysis | Mutation status | ENG | |
| Genetic Analysis | Mutation status | SMAD1 | |
| Genetic Analysis | Mutation status | SMAD4 | |
| Genetic Analysis | Mutation status | SMAD9 | |
| Genetic Analysis | Mutation status | CAV1 | |
| Genetic Analysis | Mutation status | KCNK3 | |
| Genetic Analysis | Mutation status | EIF2AK4 | |
| Genetic Analysis | Mutation status | SOX17 | |
| Genetic Analysis | Mutation status | GDF2 | |
| Genetic Analysis | Mutation status | ATP13A3 | |
| Genetic Analysis | Mutation status | AQP1 | |
| Genetic Analysis | Mutation status | KDR | |
| Genetic Analysis | Mutation status | TBX4 | |
| Biomaterial availability | Date in number of months since diagnosis | ||
| Biomaterial availability | Specimen type | Serum available | |
| Biomaterial availability | Specimen type | Plasma available | |
| Biomaterial availability | Specimen type | Whole blood available | |
| Biomaterial availability | Specimen type | Urine available | |
| Medication/PH specific drug therapy | Start drug treatment (number of months since diagnosis) | ||
| Medication/PH specific drug therapy | Stop drug treatment (number of months since diagnosis) | ||
| Medication/PH specific drug therapy | Calcium channel antagonists | Amlodipine | |
| Medication/PH specific drug therapy | Calcium channel antagonists | Diltiazem | |
| Medication/PH specific drug therapy | Calcium channel antagonists | Felodipine | |
| Medication/PH specific drug therapy | Calcium channel antagonists | Isradipine | |
| Medication/PH specific drug therapy | Calcium channel antagonists | Lercanidipine | |
| Medication/PH specific drug therapy | Calcium channel antagonists | Nifedipine | |
| Medication/PH specific drug therapy | Calcium channel antagonists | Verapamil | |
| Medication/PH specific drug therapy | Endothelin receptor antagonists | Ambrisentan | |
| Medication/PH specific drug therapy | Endothelin receptor antagonists | Bosentan | |
| Medication/PH specific drug therapy | Endothelin receptor antagonists | Macitentan | |
| Medication/PH specific drug therapy | Endothelin receptor antagonists | Sitaxentan | |
| Medication/PH specific drug therapy | PDE5 inhibitors | Sildenafil | |
| Medication/PH specific drug therapy | PDE5 inhibitors | Tadalafil | |
| Medication/PH specific drug therapy | PDE5 inhibitors | Vardenafil | |
| Medication/PH specific drug therapy | Prostacyclin PGI2-containing product | Beraprost | |
| Medication/PH specific drug therapy | Prostacyclin PGI2-containing product | Iloprost | |
| Medication/PH specific drug therapy | Prostacyclin PGI2-containing product | Epoprostenol | |
| Medication/PH specific drug therapy | Prostacyclin PGI2-containing product | Treprostinil | |
| Medication/PH specific drug therapy | Prostacyclin receptor agonists | Selexipag | |
| Medication/PH specific drug therapy | sGC-stimulators | Riociguat | |
| Medication/PH specific drug therapy | Protein-tyrosine kinase inhibitors | Imatinib | |
| Anticoagulation | Start of therapy | ||
| Anticoagulation | End of therapy | ||
| Anticoagulation | Type of anticoagulant | Phenprocoumon | |
| Anticoagulation | Type of anticoagulant | Warfarin | |
| Anticoagulation | Type of anticoagulant | INR goal (only for Marcumar and Coumadin) | |
| Anticoagulation | Type of anticoagulant | Apixaban | |
| Anticoagulation | Type of anticoagulant | Rivaroxaban | |
| Anticoagulation | Type of anticoagulant | Edoxaban | |
| Anticoagulation | Type of anticoagulant | Dabigatran |
The GoDeep Registry welcomes all PH referral centers worldwide. To participate, please e-mail us: contact@pvri-godeep-registry.org. The participating centers that currently contribute to the GoDeep registry are illustrated in the map and the tables below:
Contributing Members
The PVRI GoDeep team
Medical Team: Werner Seeger, Khodr Tello, Athiththan Yogeswaran, Patrick Janetzko
IT and Data Warehouse Configuration: Raphael Majeed, Kurt Marquardt, Achim Michel-Backofen
Data Documentation: Philipp Krieb, Aileen Müller, Farhan Mubashir
Statistics and Artificial Intelligence Analyses: Meike Fuenderich, Jochen Wilhelm
Giessen Contact Information
E-Mail: contact@pvri-godeep-registry.org
Telephone +49-641-985-42302
Legal notice
Information in accordance with section 5 TMG.
PVRI GoDeep Registry
Director Prof. Werner Seeger
Institute for Lung Health, Justus Liebig University Giessen
Gaffkystr.9
35392 Giessen
Germany
Our site uses cookies and similar technologies. By using the site, you consent to the use of cookies.
You can find more information on this in our Privacy Policy.